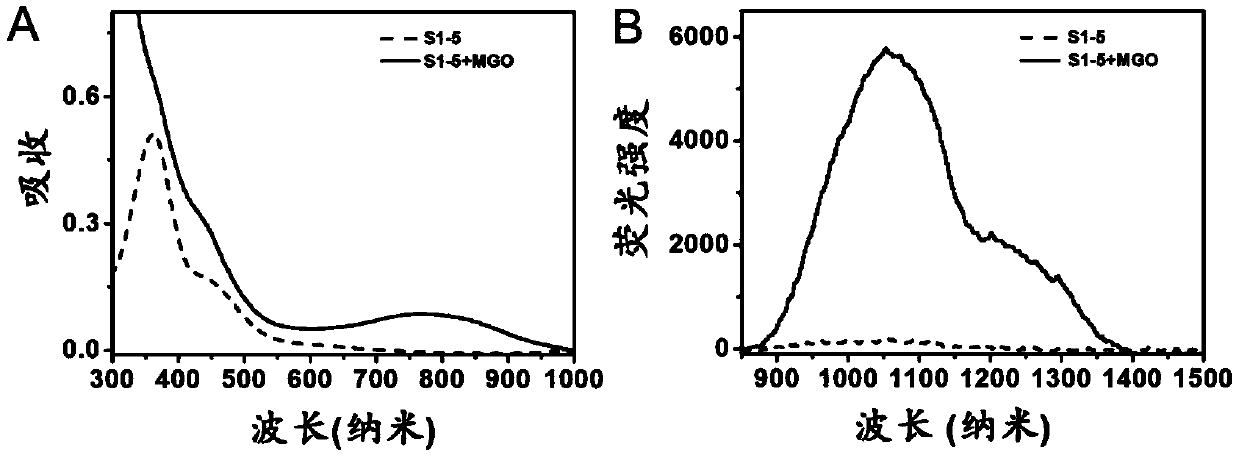
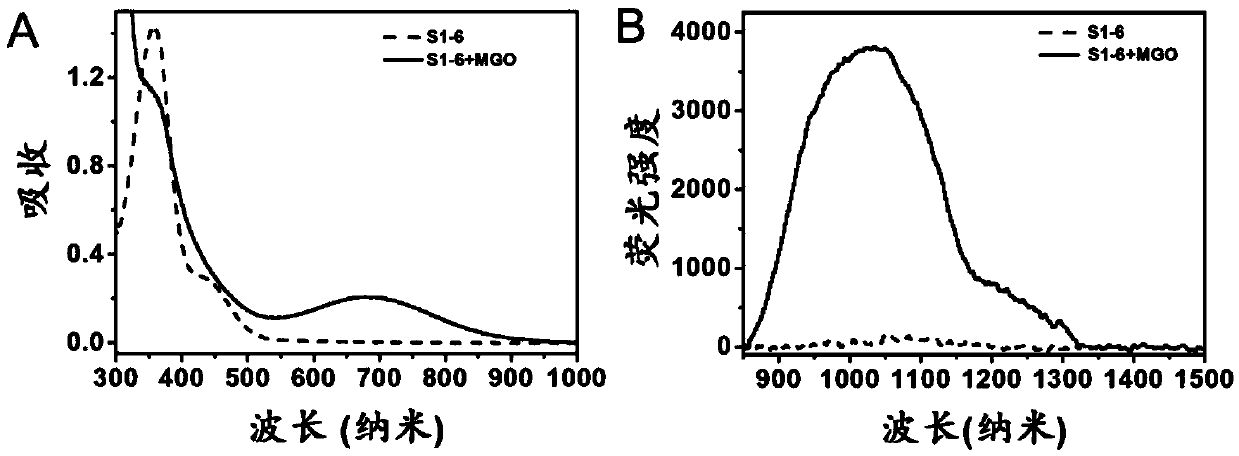
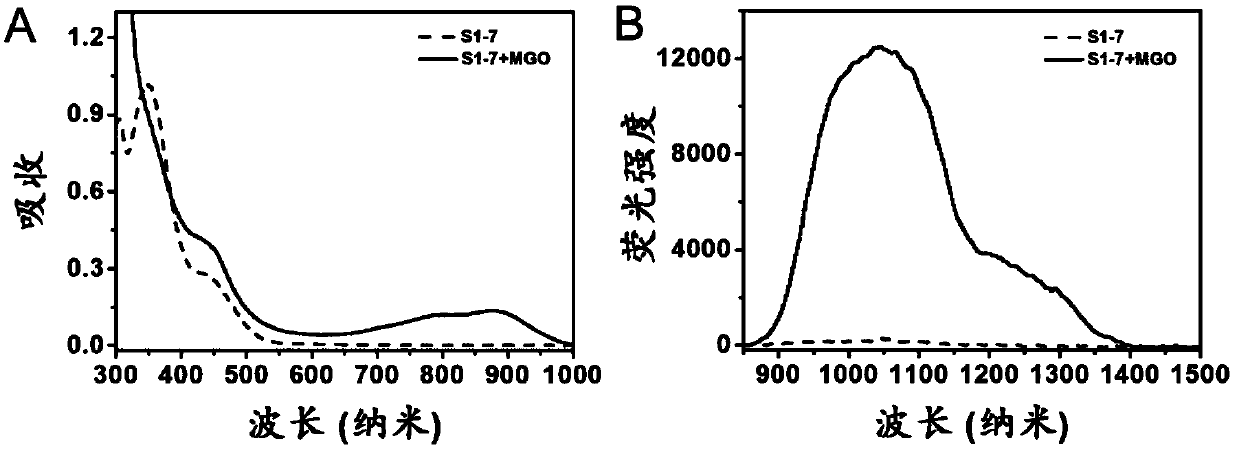
Compound, preparation method and application thereof as near-infrared region-II fluorescent probe for detecting pyruvaldehyde
A compound, polyethylene glycol-based technology, applied in the field of compounds, can solve the problems of complicated operation, narrow application range, and cell lysis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1, the synthesis of compound S1-1
[0059]
[0060] Compound C1 was synthesized by reference method (Chem. Sci., 2016, 7:6203-6207). Then compound C1 (100 mg, 0.06 mmol) was dissolved in 3 mL of acetic acid, Fe powder (100 mg, 1.79 mmol) was added, and the temperature of the reaction solution was raised to 100° C., and reacted for 6 h under nitrogen protection. After the reaction was completed, the pH value of the reaction solution was adjusted to 7-8 with saturated sodium bicarbonate solution, extracted three times with dichloromethane, and the organic phases were combined. Washed with saturated brine and dried over anhydrous sodium sulfate. After suction filtration, the filtrate was concentrated under reduced pressure, and the crude product was separated by column chromatography to obtain 55 mg of yellow solid compound S1-1, with a yield of 57%. 1 HNMR (300MHz, CDCl 3 )δ7.50(d, J=8.49 Hz, 4H), 7.32(m, 4H), 7.07(m, 20H), 4.19(t, J=8.43Hz, 8H), 2.92(m, ...
Embodiment 2
[0061] Embodiment 2, the synthesis of compound S1-2
[0062]
[0063] Compound S1-1 (10 mg, 0.0066 mmol) was dissolved in an appropriate amount of dichloromethane, added trifluoroacetic acid (dichloromethane: trifluoroacetic acid = 10:1, v / v), and stirred at room temperature for 5 h. The reaction solution was concentrated under reduced pressure, and the crude product was separated by column chromatography to obtain 4 mg of compound S1-2 as a yellow solid, with a yield of 54%. ESI-MS theoretical value is C 62 h 52 N 6 o 8 S 3 :1104.3, measured value is 1105.3[M+H] + .
Embodiment 3
[0064] Embodiment 3, the synthesis of compound S1-3
[0065]
[0066] Compound A2 was synthesized in two steps: 1,4-dibromo-2,3-dinitrobenzothiazole (100.0mg, 0.26mmol), tributyl (2,3-dihydrothieno[3,4-B ]-[1,4]dioxin-5-yl)stannane (336.0 mg, 0.78 mmol) and bistriphenylphosphine palladium dichloride (52.0 mg, 0.074 mmol) were added to 8.0 mL of distilled toluene, Nitrogen protection, reflux reaction for 12h. After the reaction was detected by TLC, water and ethyl acetate were added for extraction, and the organic layer was washed with water and saturated brine in sequence, and dried over anhydrous sodium sulfate. After suction filtration, the filtrate was concentrated under reduced pressure, and the crude product was separated by column chromatography to obtain 126.6 mg of compound i as an orange solid, with a yield of 96%. 1 HNMR (300MHz, CDCl 3 )δ6.77(s,2H), 4.22(dd,J=11.5,5.5Hz,8H). 13 CNMR (125MHz, CDCl 3 )δ152.63, 143.07, 142.52, 141.21, 120.28, 105.49, 104.61, 64...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com