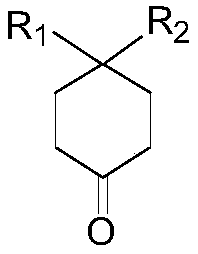
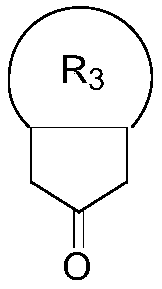
Novel method for synthesizing isotopic hydroxyl and methylsulfonyl methyl compound
A technology of methanesulfonylmethyl and iso-hydroxyl, which is applied in the field of organic compound synthesis, can solve the problems of unfavorable environmental protection of waste liquid, poor stability of intermediates, low conversion rate and yield, and achieve short steps, mild reaction conditions, The effect of lowering the security level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1. Preparation of 8-(methylsulfonylmethyl)-1,4-dioxospiro[4,5]decane-8-ol
[0049]
[0050] Add 0.5g of dimethyl sulfone and 6mL of tetrahydrofuran to the reaction vessel, replace the reaction system with argon and then stir and cool to -70℃, add 1.4mL of n-butyllithium with a concentration of 2.5mol / L and keep the temperature at -70℃ Stir for 0.5 hours; then add 0.4 g of 1,4-cyclohexanedione monoethylene ketal (S-1) in tetrahydrofuran (6 mL) into the reaction system, keep the temperature of the reaction solution at -70°C and stir for 2 hours, Warm to room temperature and stir for 2 hours; the reaction solution is extracted with saturated aqueous ammonium chloride solution, extracted with ethyl acetate, the combined organic phases are washed with saturated brine, dried with anhydrous sodium sulfate, filtered, and the filtrate is spin-dried at low temperature to remove the solvent and residue Column chromatography was separated and purified to obtain the off-white s...
Embodiment 2
[0054] Example 2. Preparation of 5-hydroxy-5-(methylsulfonylmethyl)hexahydropentene-2(1H)-one
[0055]
[0056] Add 0.5 g of dimethyl sulfone and 6 mL of tetrahydrofuran to the reaction vessel, replace the reaction system with argon, stir and cool to -75°C, add 1.4 mL of n-butyl lithium with a concentration of 2.5mol / L, and keep the temperature at -75°C Stir for 0.5 hours; then add 0.5g of bicyclo[3.3.0]octane-3,7-dione (S-2) in tetrahydrofuran (7mL) solution into the reaction system, keep the temperature of the reaction solution at -75℃ and stir for 2 hours , Warmed to room temperature and stirred for 3 hours; the reaction solution was extracted with a saturated aqueous solution of ammonium chloride, extracted with ethyl acetate, the combined organic phases were washed with saturated brine, dried with anhydrous sodium sulfate, filtered, and the filtrate was spin-dried at low temperature to remove the solvent. The residue was separated and purified by column chromatography to obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com