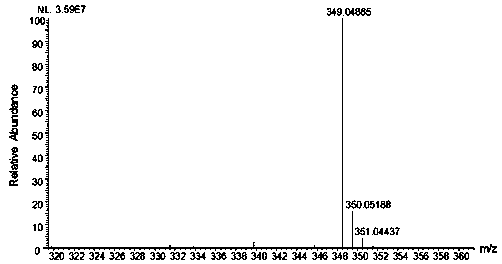
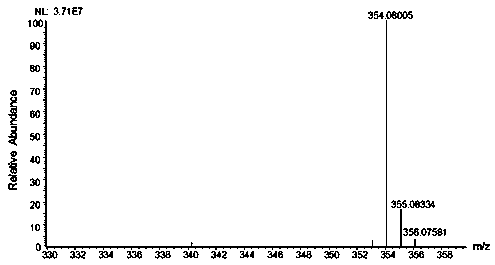
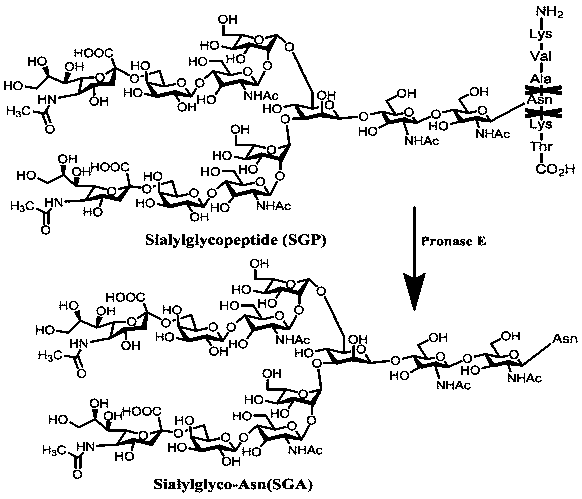
Sugar chain relative quantitative isotope labeling mass spectrum derivatization reagent
A technology for quantitative isotopes and derivatization reagents, applied in the field of biological analysis, can solve the problems of difficult synthesis, difficulty in obtaining, expensive, etc., and achieve high selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] Synthesis of d0-DPBOC:
[0031] L-2-Thiazolidinone-4-carboxylic acid (L-2-Thiazolidinone-4-carboxylic-acid, OTZD), 100.0 mg dissolved in 2.0 mL N,N- Add 184.6 μL of triethylamine to dimethylformamide (DMF), slowly add 92.4 μL of benzoyl chloride (d0) dropwise under the condition of stirring in an ice bath, and stop the reaction after stirring in an ice bath for 2 h. Add 10 mL of water, adjust the pH value of the reaction solution with acid, add the same volume of ethyl acetate, and after fully standing still, discard the water layer. Then add an equal volume of ethyl acetate, repeat the extraction 3 times, combine the ethyl acetate layers and dry over anhydrous sodium sulfate, filter with suction, add silica gel and spin dry, and separate by column chromatography to obtain 98.3 mg of white solid with a yield of about It was 59.17%.
[0032] d0-BZC-OTZD 92.5 mg, add 8 mL of dichloromethane (DCM) to dissolve, then add 52.2 mg of NHS, dicyclohexylcarbodiimide (DCC) 93.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com