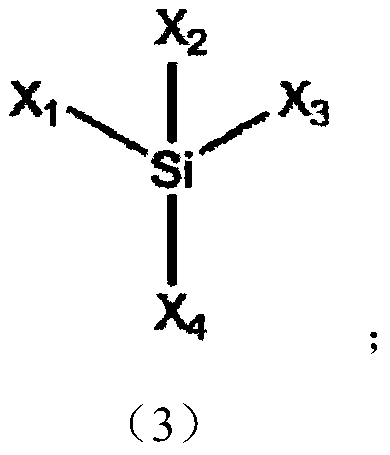
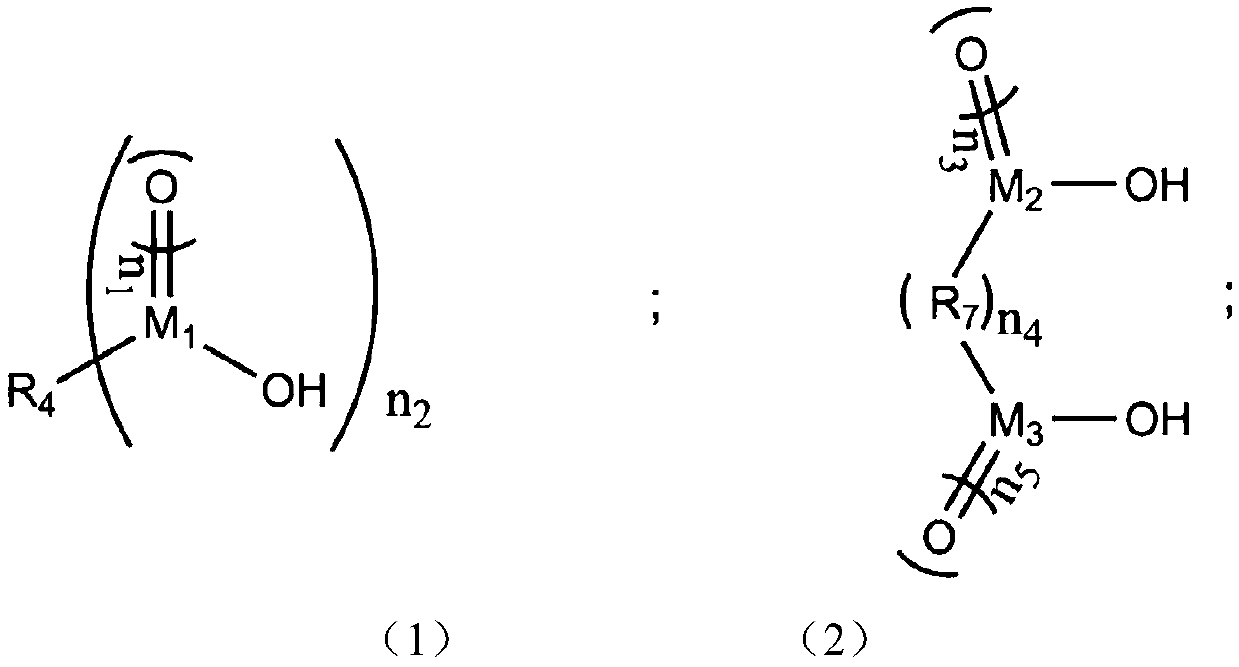Preparation method of silicon-based ester compound, silicon-based ester compound, electrolyte containing silicon-based ester compound and secondary battery
An amine compound and compound technology are applied in the fields of silicon-based ester compound preparation, silicon-based ester compound, electrolyte containing the same, and secondary battery, and can solve the problems of complicated operation process, difficult handling and purification, complicated operation, etc. , to achieve the effects of convenient operation in the preparation process, long reaction time and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
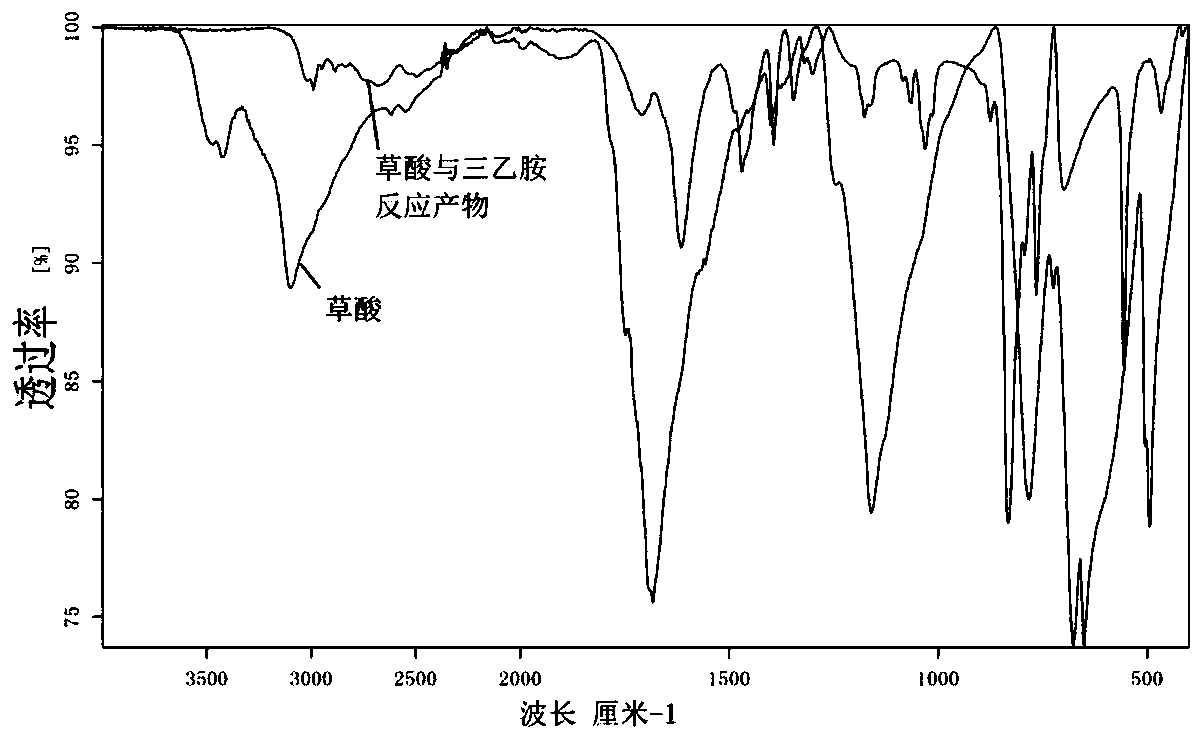
[0067] Add oxalic acid (0.2mol) and DMSO (150mL) to the flask, then add monomethylamine (0.44mol), react at room temperature for 1h overnight and let stand for 12h; then add trimethylbromosilane (0.22mol), then add DMSO (130ml) , heated at 70° C. for 8 h; after the reaction, suction filtration and vacuum distillation (20 mmHg) gave white solid bis(trimethylsilyl) oxalate with a purity of 97% and a yield of 81%.
Embodiment 2
[0069] Add oxalic acid (0.15mol) and acetonitrile (150mL) to the flask, add trimethylamine (0.05mol), and react at room temperature for 1h; then add dimethylvinylchlorosilane (0.15mol), and heat at 50°C for 2h; Suction filtration and vacuum distillation (20mmHg) after the end, to obtain a colorless liquid bis(dimethylvinylsilyl) oxalate with a purity of 97% and a yield of 85%.
Embodiment 3
[0071] Add oxalic acid (0.2mol) and carbon tetrachloride (150mL) to the flask, then add monopropylamine (0.44mol), and react at room temperature for 1h; then add dimethylisopropylbromosilane (1mol), and heat the reaction at 100°C 6h; after the reaction, suction filtration and vacuum distillation (20mmHg) gave a colorless liquid bis(dimethylisopropylsilyl) oxalate with a purity of 97% and a yield of 55%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com