Degradable anti-infection and anti-calculus fe-cu alloys suitable for urinary implant materials
An implant material, anti-infection technology, applied in medical science, prosthesis, etc., can solve the problems of urinary tract infection and calculus, increase retrograde infection, disrupt the normal operation system, etc., to inhibit adhesion and proliferation, prevent calculus formation. , the effect of strong antibacterial function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] In this embodiment, the composition of the Fe-0.5Cu alloy is: 0.5% Cu, and the balance is Fe.
[0040] Preparation method: The above-mentioned composition alloy is made into an ingot by vacuum induction melting, and after being kept at 1000°C for 2 hours, it is forged into a rod of Φ20mm, processed into a rod of Φ6mm~10mm by extrusion or swaging, and processed into a rod of Φ0. 2 ~ 0.6mm wire, prepared into staples (YY / T 0245-2008). During the drawing process, supplemented by heat treatment, the treatment process is 1000 ° C under vacuum atmosphere for 1 hour, water quenching and cooling to room temperature.
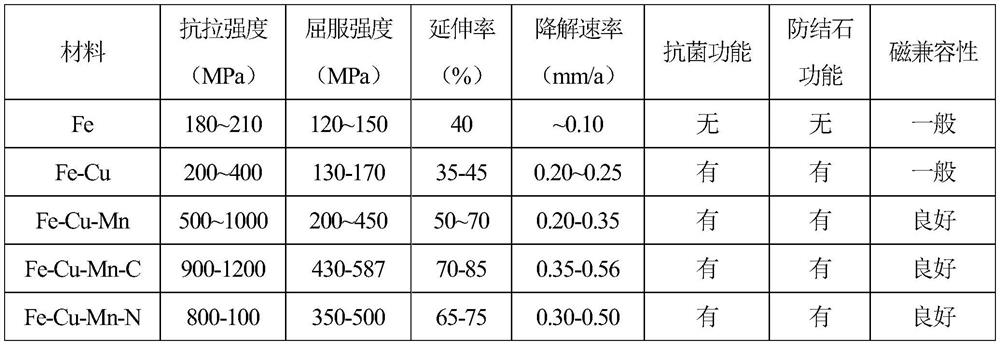
[0041] Tensile properties (GB / T 228-2002): The tensile strength is 200MPa, the yield strength is 130MPa, and the elongation is 45%.
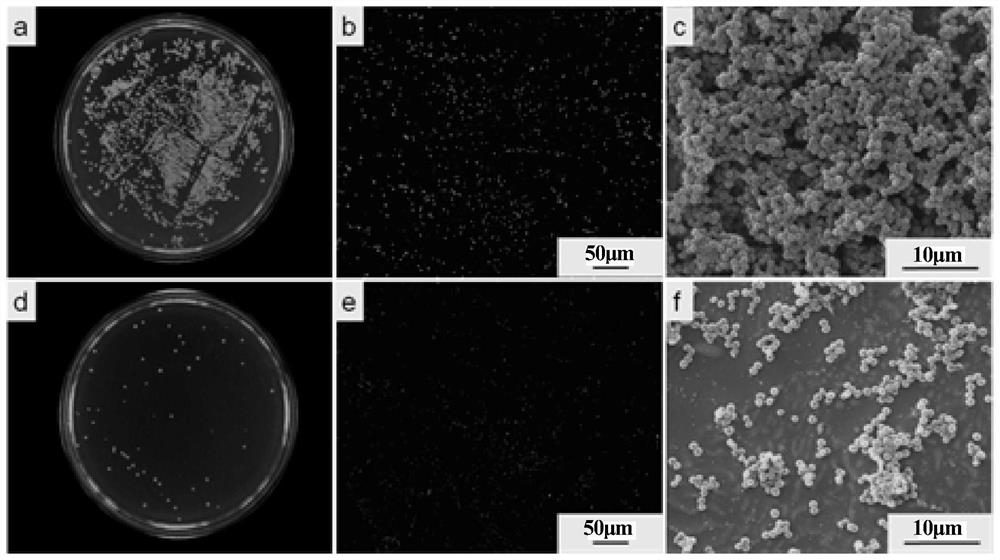
[0042] Antibacterial properties (GB / T 2591): Sterilization rate for Staphylococcus aureus: 98%; Sterilization rate for Mirabilis proteus: 95%.
[0043] Anti-stone performance: stone rate <5%.
[0044] Degradation rate (artificial...
Embodiment 2
[0047] In this embodiment, the composition of the Fe-1Cu-25Mn alloy is: 1% Cu, 25% Mn, and the balance is Fe.
[0048] Preparation method: The above-mentioned composition alloy is made into an ingot by vacuum induction melting, and after being kept at 1000°C for 2 hours, it is forged into a rod of Φ20mm, processed into a rod of Φ6mm~10mm by extrusion or swaging, and processed into a rod of Φ0. 2-1mm wire, prepared into staples (YY / T 0245-2008). During the drawing process, supplemented by heat treatment, the treatment process is 1000 ° C under vacuum atmosphere for 1 hour, then water quenching.
[0049] Tensile properties (GB / T 228-2002): The tensile strength is 820MPa, the yield strength is 260MPa, and the elongation is 65%.
[0050] Antibacterial properties (GB / T 2591): Sterilization rate for Staphylococcus aureus: 99%; Sterilization rate for Mirabilis proteus: 96%.
[0051] Anti-stone performance: stone rate <5%.
[0052] Degradation rate (artificial urine, soaked for 30 ...
Embodiment 3
[0055] In this embodiment, the composition of the Fe-2Cu-20Mn-1C alloy is: 2% Cu, 20% Mn, 1% C, and the balance is Fe.
[0056] Preparation method: The above composition alloy is made into an ingot by vacuum induction melting, and after being kept at 980°C for 3 hours, it is forged into a rod of Φ20mm, processed into a rod of Φ6mm~10mm by extrusion or swaging, and processed into a rod of Φ0. 2-1mm wire, prepared into staples (YY / T 0245-2008). During the drawing process, supplemented by heat treatment, the treatment process is 950 ° C for 1.5 hours in a vacuum atmosphere, and then water quenching.
[0057] Tensile properties (GB / T 228-2002): The tensile strength is 1050MPa, the yield strength is 360MPa, and the elongation is 70%.
[0058] Antibacterial properties (GB / T 2591): Sterilization rate for Staphylococcus aureus: 99%; Sterilization rate for Mirabilis proteus: 99%.
[0059] Anti-stone performance: stone rate <5%.
[0060] Degradation rate (artificial urine, soaked for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| yield strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com