Injectable hyaluronic acid hydrogel for cartilage repair and preparation method of injectable hyaluronic acid hydrogel
A technology for hyaluronic acid and cartilage repair, which is used in pharmaceutical formulations, medical science, prostheses, etc. It can solve the problems of low inducibility of biomaterials, high risk of immune rejection, and high treatment costs, optimize graft concentration, and reduce surgery. Number of times, significant social and economic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
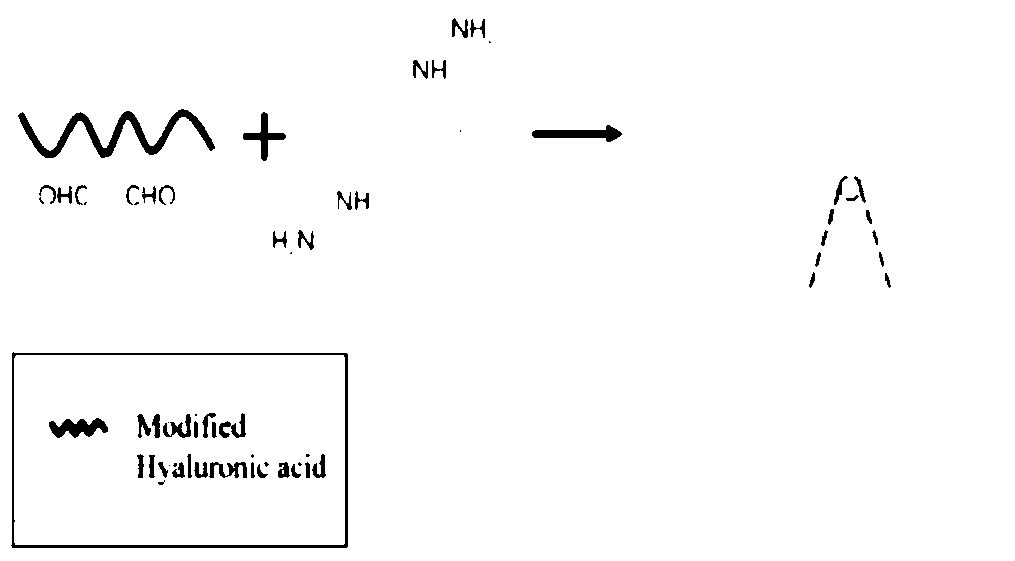
[0066] Step 1: dissolving sodium hyaluronate in water at room temperature to form a transparent solution with a mass fraction of 0.1%, adding a cation exchange resin and stirring overnight, and filtering to obtain a hyaluronic acid stock solution;
[0067] Step 2 Add periodate to the hyaluronic acid stock solution obtained in step 1, the molar ratio of the two is 10:1, place the system in the dark for 0.5 hours; add ethylene glycol to remove excess oxidant, and continue stirring for 1 hour After the reaction is terminated, the product is purified by dialysis and freeze-dried to obtain ring-opened hyaluronic acid;
[0068] Step 3: Add cross-linking agent EDC / NHS to the hyaluronic acid stock solution obtained in step 1, the molar ratio is 1:1, stir for 0.5 hours, add excess hydrazine to react for 24 hours; dialysis purification, freeze-drying to obtain hyaluronic acid grafted hydrazide acid;
[0069] Step 4 Dissolve the above two modified hyaluronic acids in PBS solution respec...
Embodiment 2
[0071] Step 1: Dissolve sodium hyaluronate in water at room temperature to form a transparent solution with a mass fraction of 3%, add cation exchange resin and stir overnight, and filter to obtain a hyaluronic acid stock solution;
[0072] Step 2 Add permanganate to the hyaluronic acid stock solution obtained in step 1, the molar ratio of the two is 1:10, and place the system in a dark place to react for 6 hours; add ethylene glycol to remove excess oxidant, and continue stirring for 1 hour After the reaction is terminated, the product is purified by dialysis and freeze-dried to obtain ring-opened hyaluronic acid;
[0073] Step 3: Add cross-linking agent EDC / NHS to the hyaluronic acid stock solution obtained in step 1, the molar ratio is 1:10, stir for 0.5 hours, add excess adipic dihydrazide, react for 24 hours; dialysis purification, freeze-drying to obtain graft hydrazide hyaluronic acid;
[0074] Step 4 Dissolve the above two modified hyaluronic acids in PBS solution res...
Embodiment 3
[0076] Step 1: dissolving sodium hyaluronate in water at room temperature to form a transparent solution with a mass fraction of 0.1%, adding a cation exchange resin and stirring overnight, and filtering to obtain a hyaluronic acid stock solution;
[0077] Step 2 Add methacrylic anhydride in an equal mass ratio to the hyaluronic acid stock solution obtained in step 1, maintain the pH of the system at 8, and react at 4°C for 12 hours to obtain hyaluronic acid modified with methacrylic acid; then add hyaluronic acid The pH of the system was adjusted to 6.0, the cross-linking agent EDC / NHS was added to the solution, stirred for 0.5 hours, the TGF-β affinity peptide HSNGLPL was added, reacted for 3 hours, purified by dialysis, and freeze-dried to obtain the grafted TGF-β affinity peptide Polypeptide hyaluronic acid.
[0078] Step 3 Add chlorate to the hyaluronic acid stock solution obtained in step 1, the molar ratio of the two is 10:1, and place the system in a dark place to reac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com