A Lectin-Magnetic Carrier Conjugated Complex for the Isolation of Glycosylated Exosomes from Clinical Samples
A magnetic carrier and exosome technology, applied in carrier binding/immobilization of peptides, analytical materials, biological testing, etc., can solve the problems of large steric hindrance, rupture of exosome vesicles, low separation efficiency, etc., reducing space The effect of steric hindrance, reducing steric hindrance and improving separation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 2
[0069] Example 2 Preparation of Glycosylated Exosome Separation Composition
[0070] The present invention provides a composition for separating glycosylated exosomes, which specifically includes: a lectin-magnetic carrier conjugated complex, which is used for washing and removing non-specifically bound and unglycosylated exosomes during the separation process. and other impurities, and / or eluate for elution of glycosylated exosomes specifically bound to lectin-magnetic carrier conjugated complexes, individually packaged and present in a set or in the kit.
[0071] In this example, the above-mentioned lectin-magnetic carrier conjugated complex is the LCA-agarose magnetic bead solution with a volume ratio of 50% obtained in Example 1.
[0072] The above cleaning solution is a metal-salt ion-free cleaning buffer or purified water, and can optionally be a metal-salt-free cleaning buffer, such as: the main component includes 10-200mM metal-salt-free TRIS-HCl buffer with a pH of 7...
Embodiment example 3
[0074] Example 3 Method of using glycosylated exosome isolation composition
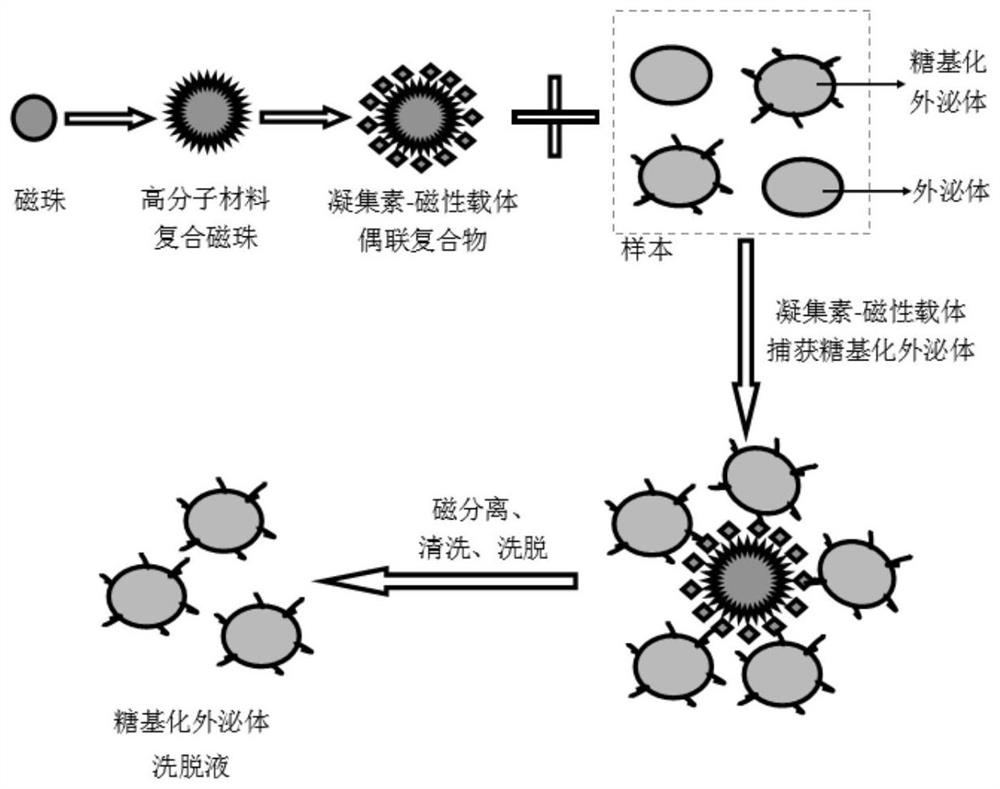
[0075] The present invention also provides a method for separating glycosylated exosomes, which mainly includes the main experimental steps of using the composition for separating glycosylated exosomes in Example 2. Please refer to the schematic diagram of the separation principle for details. figure 1 "Schematic diagram of the separation of glycosylated exosomes by lectin-magnetic carrier conjugated complexes".
[0076] The detailed experimental steps of the present invention include:
[0077] 1. Preparation before the experiment
[0078] Self-provided equipment or equipment: magnetic stand for manual separation of glycosylated exosomes; or use the fully automatic agarose magnetic bead separation instrument of the group company and its subsidiaries, mainly for automatic glycosylated exosome separation separation, to achieve the purpose of saving manpower. The automatic separation of glycosylated ...
Embodiment example 4
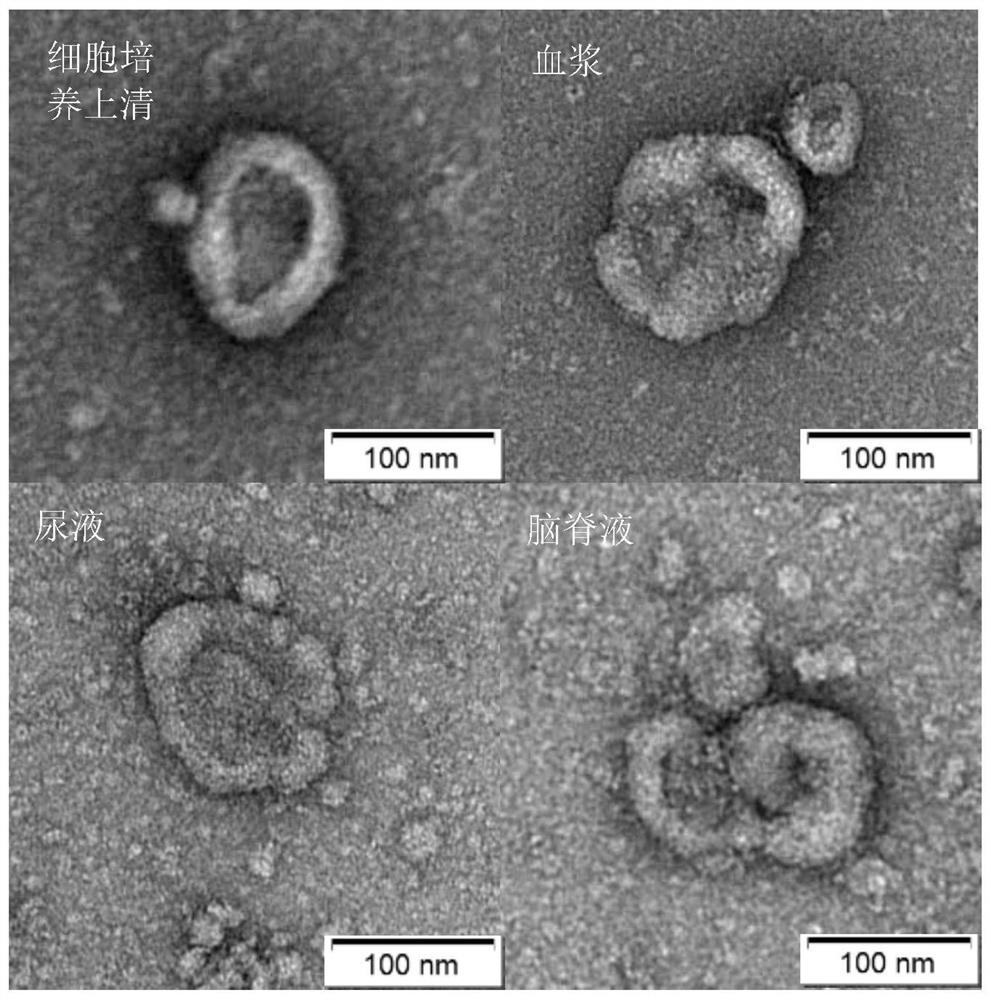
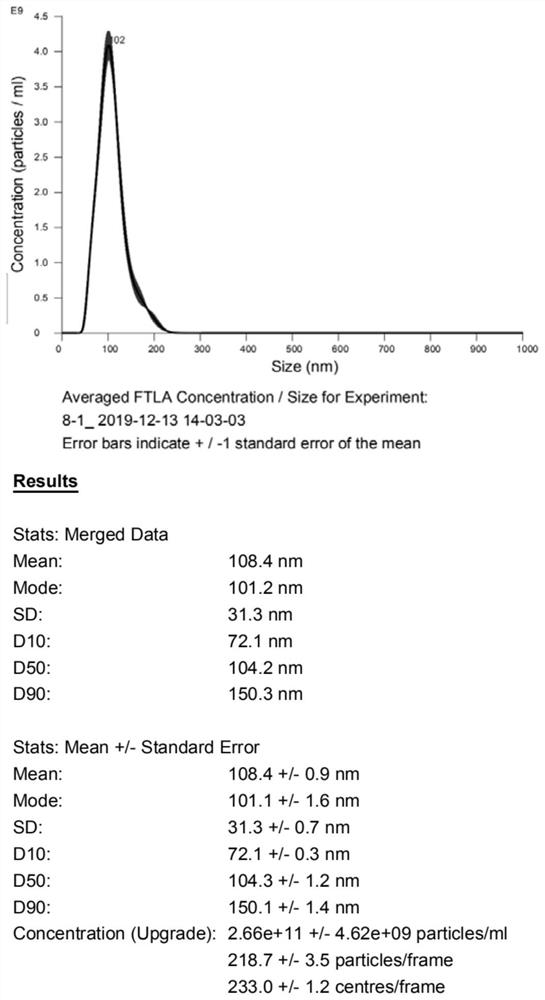
[0087] This example mainly further illustrates the separated samples of glycosylated exosomes. The samples to be separated that can be used in the present invention are mainly: serum, plasma, saliva, tissue or cell culture supernatant, urine, cerebrospinal fluid, lymph fluid any one; for conventional 1.5ml centrifuge tube, add the pretreated sample volume 50-500ul of the serum, plasma, saliva, cerebrospinal fluid, lymph, tissue or cell culture supernatant, urine, preferably 50 -300ul, more preferably 100-200ul; in the sample pretreatment process, the samples of serum, plasma, saliva, cerebrospinal fluid, and lymph are only centrifuged, so for 50-500ul, 50-300ul can be selected, and 100 can be selected. -200ul of the sample can be pre-treated to obtain the required volume of pre-treated samples; and the tissue or cell culture supernatant, urine samples in the sample pre-treatment process, in addition to the centrifugation step, also need Concentrate 10-1000 times, so in order t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com