Folic acid modified tellurium bond bridged polyethylene glycol-poly(epsilon-caprolactone) segmented copolymer as well as preparation method and application thereof
A technology of block copolymer and polyethylene glycol, which is applied in the field of polyethylene glycol-polyblock copolymer drug delivery system and nano-drug delivery system, which can solve the problem of low responsiveness and insufficient responsiveness of nano-delivery system and other problems, to achieve the effect of simple and controllable synthesis method, which is conducive to repetition and improves the therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
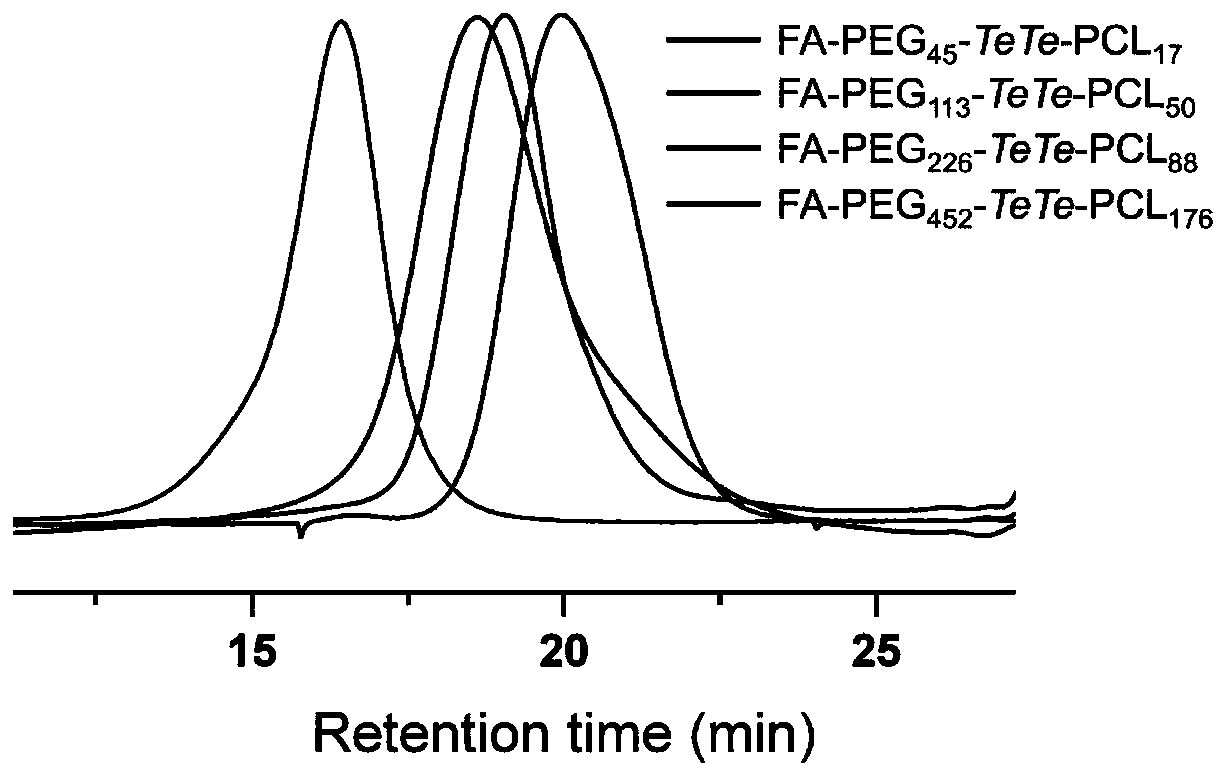
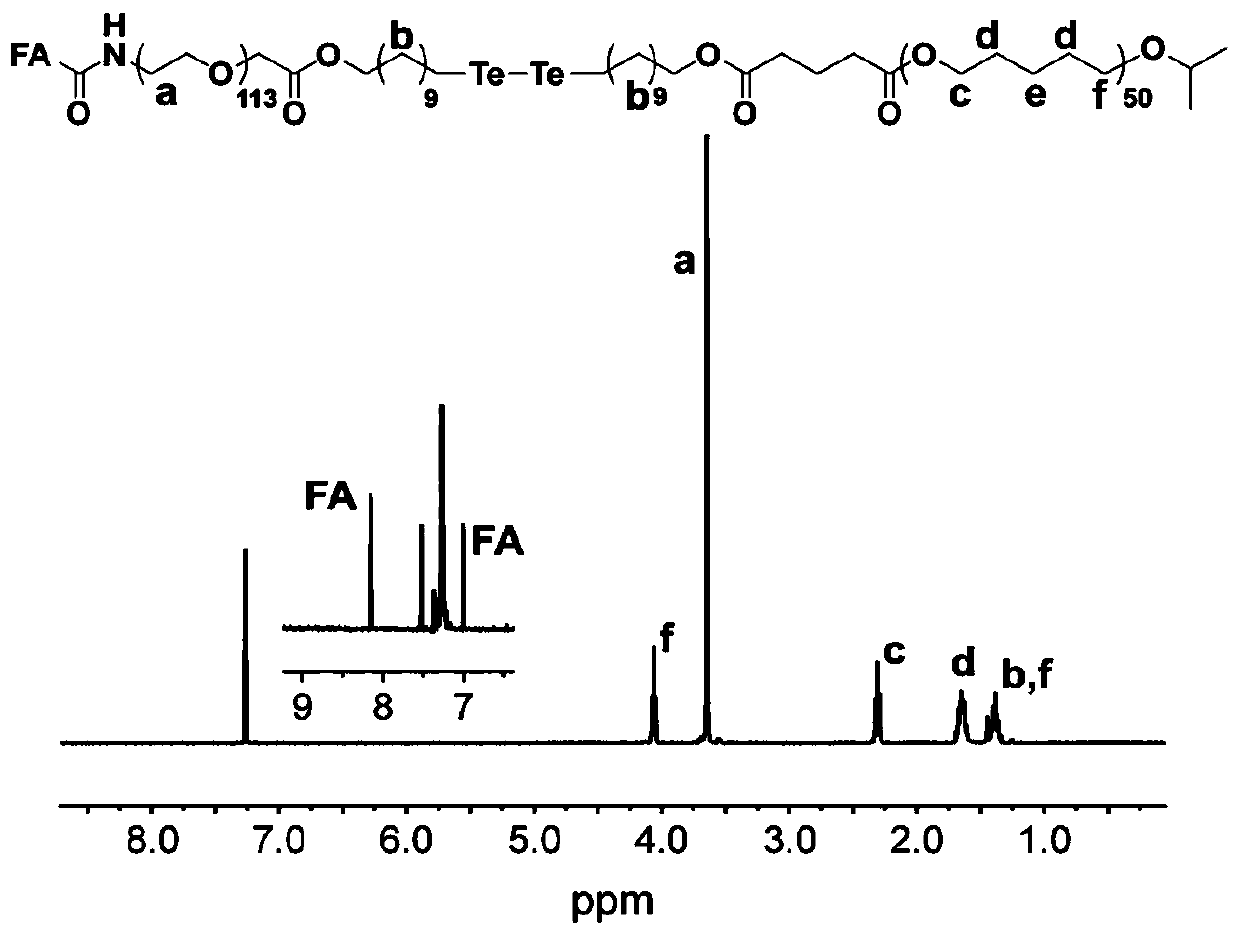
[0087] Example 1: Synthesis of folic acid modified ditellurium bond bridged polyethylene glycol-poly(ε-caprolactone) block copolymer
[0088] The chemical structure and synthetic route of folic acid modified ditellurium bond bridged polyethylene glycol-poly(ε-caprolactone) block copolymer are shown in the appendix figure 1 shown.
[0089] Folate-modified ditellurium bond-bridged polyethylene glycol-poly(ε-caprolactone) block copolymers with different molecular weights are polyethylene glycol derivatives with ditellurium bonds and folic acid groups at the end groups and terminal groups For carboxyl poly (ε-caprolactone) coupled. The specific experimental steps of synthesis are as follows:
[0090] At 25°C, completely dissolve FA-PEG-TeTe-OH, PCL-COOH, dicyclohexylcarbodiimide and 4-dimethylaminopyridine in anhydrous dichloromethane according to the ratio in Table 2, and react for 24 hours . After the reaction, by-products were removed by filtration, precipitated three times...
Embodiment 2
[0098] Embodiment 2: nanoparticle preparation
[0099] Amphiphilic polyethylene glycol-poly(ε-caprolactone), based on the hydrophobic-hydrophobic interaction in aqueous solution, can obtain "core-shell" micellar structure nanoparticles in water by optimizing the preparation method under specific conditions . In its hydrophobic core, hydrophobic drug molecules can be entrapped to obtain a nano drug delivery system. In this example, the nanoprecipitation method was used to prepare the following nanoparticles.
[0100] 1) Preparation of unloaded nanoparticles with FA-PEG 113 -TeTe-PCL 50 As an example, the specific method is: FA-PEG with a mass of 10mg 113 -TeTe-PCL 50 Dissolve in 200 μL of DMSO, slowly drop the above oil phase into 5 mL of stirred ultrapure water, continue to stir for 30 minutes after the dropwise addition, transfer to a dialysis bag with a molecular weight cut-off of 3500, and use ultrapure water for dialysis for 24 hours at 3000 rpm Empty nanoparticles w...
Embodiment 3
[0102] Example 3: DOX release assay of ditellurium bond bridged targeted nano-delivery system under different conditions
[0103] This embodiment selects mPEG 113 -b-PCL 52 、FA-PEG 113 -b-PCL 46 and FA-PEG 113 -TeTe-PCL 50 Preparation of drug-loaded nanoparticles, respectively named as NP DOX , F-NP DOX and F-TeNP DOX . Dilute the nanoparticle solution to 2 mL (10 mg / mL) with a pH 7.40 buffer solution containing glutathione, transfer to a dialysis tube with a molecular weight cut-off of 3500, immerse in 15 mL of buffer solution, and process on a shaking table at 37 ° C and 60 rpm . At different time intervals, take 100 μL of the buffer solution outside the dialysis bag, and detect the DOX content in the solution by high performance liquid chromatography. The results are shown in Figure 5 .
[0104] Depend on Figure 5 As shown, for NPs without ditellurium bonds DOX , F-NP DOX The release behavior of DOX is basically the same no matter whether glutathione is adde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com