Method for preparing macro-monomer synthesized polycarboxylate water reducer through alcoholysis of alkenyl-acyl chloride
A technology for synthesizing polycarboxylic acid and alkenyl chlorohydrin, which is applied in the field of polycarboxylic acid high-performance water reducer for concrete, can solve the problems of failure to achieve functional regulation of chemical admixtures, limit popularization and application, and achieve extreme side effects , expanding the theoretical connotation, and the effect of mild and stable polymerization reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
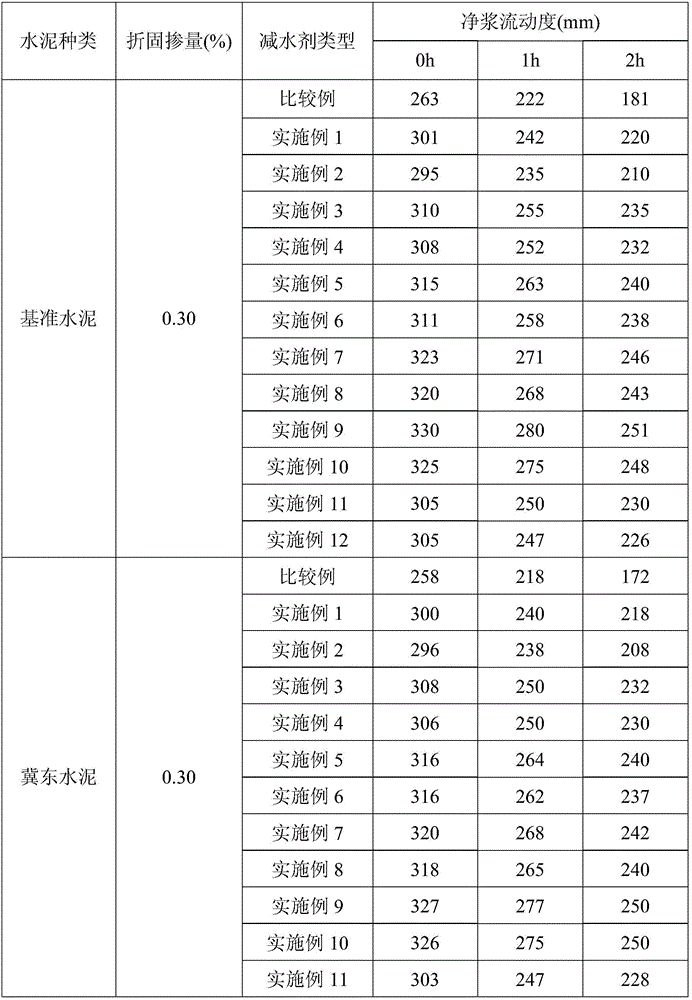
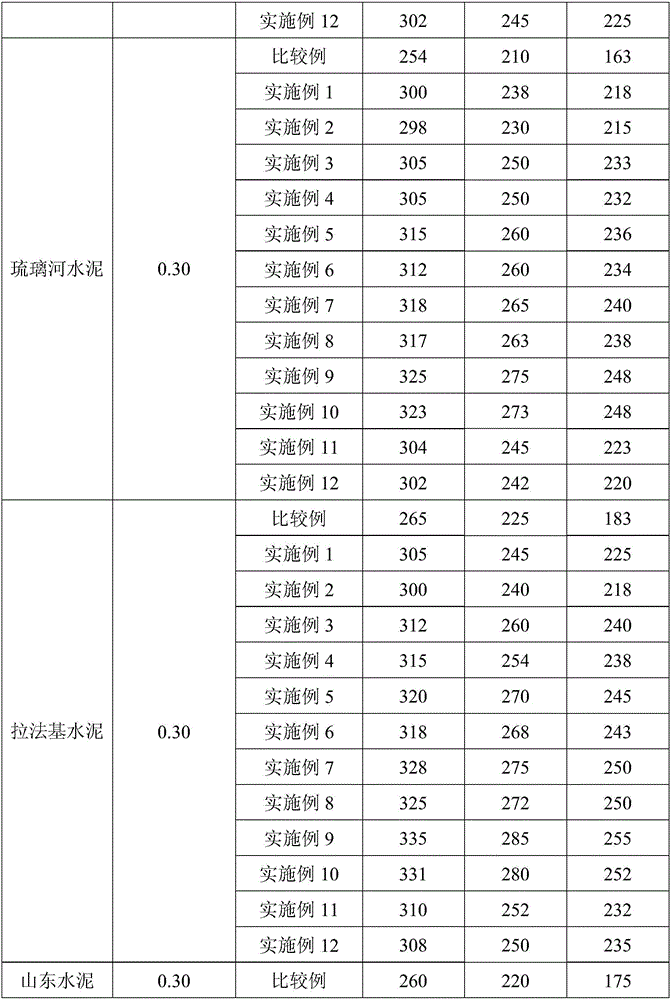
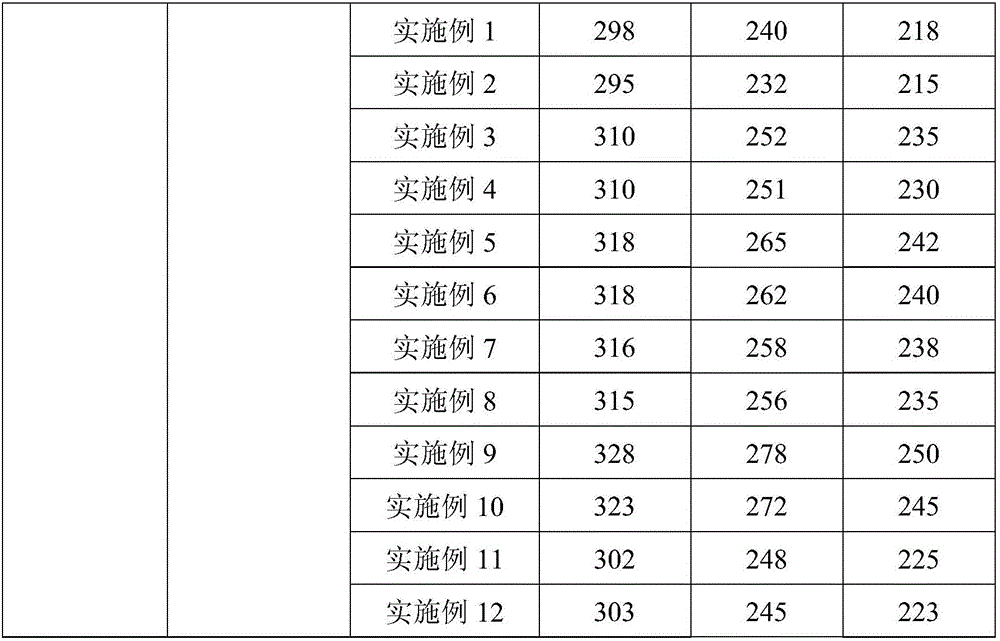
Image
Examples
Embodiment 1
[0027] First, 22.07g of methacryloylpropyltrimethylammonium chloride and 1g of cyclohexanol were added to the reactor, and then 553.81g of water was added to prepare an aqueous solution with a mass concentration of 4%, and the reactor was filled with nitrogen to remove oxygen repeatedly 3 times Seal it after 18 minutes, add 5.97g of ceric ammonium sulfate, stir for 18 minutes until it is mixed evenly, continue to heat up to 10°C for polymerization reaction, and react for 16 hours to obtain an aqueous solution of hydroxyl-terminated cation side chains; The aqueous solution was vacuumed to remove the water in the system, and 25g of polyethylene glycol monomethyl ether (molecular weight=500), 5.43g of acryloyl chloride, and 262.52g of dimethylformamide were sequentially added, and the stirring time was 10 minutes between each addition, and the temperature was raised to 40 ℃, after 4 hours of reaction, add 420.03g of methanol, wash the precipitate repeatedly 5 times, vacuum dry the...
Embodiment 2
[0029] After the polycarboxylate superplasticizer solution with a mass fraction of 30% obtained in Example 1 was stored at 6° C. for 20 days, its implementation effect was measured.
Embodiment 3
[0031] First, 14.71g of methacryloyloxyethyl dimethyl butyl ammonium bromide and 0.74g of isobutanol were added to the reactor, then 757.11g of water was added to prepare an aqueous solution with a mass concentration of 2%, and the reactor was filled with nitrogen repeatedly After 4 times of deoxygenation for 30 minutes, seal it, add 0.6g of ammonium cerium sulfate, stir for 30 minutes until it is evenly mixed, continue to heat up to 55°C for polymerization reaction, and react for 6 hours to obtain an aqueous solution of hydroxyl-terminated cation side chains; The hydroxyl cation side chain aqueous solution was vacuumed to remove the water in the system, and 45g polyethylene glycol monomethyl ether (molecular weight = 3000), 3.66g methacryloyl chloride, 253.47g dimethyl sulfoxide were added in sequence, and the stirring time between each addition was After 12 minutes, the temperature was raised to 85°C. After 2.5 hours of reaction, 633.69g of methanol was added, the precipitate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com