Galactosylated chitosan coated mesoporous silica carrier and application thereof
A technology of mesoporous silica and chitosan, which is applied to medical preparations containing no active ingredients, medical preparations containing active ingredients, and the digestive system, can solve problems such as unstable oral bioavailability, and achieve The effect of increasing the intake rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
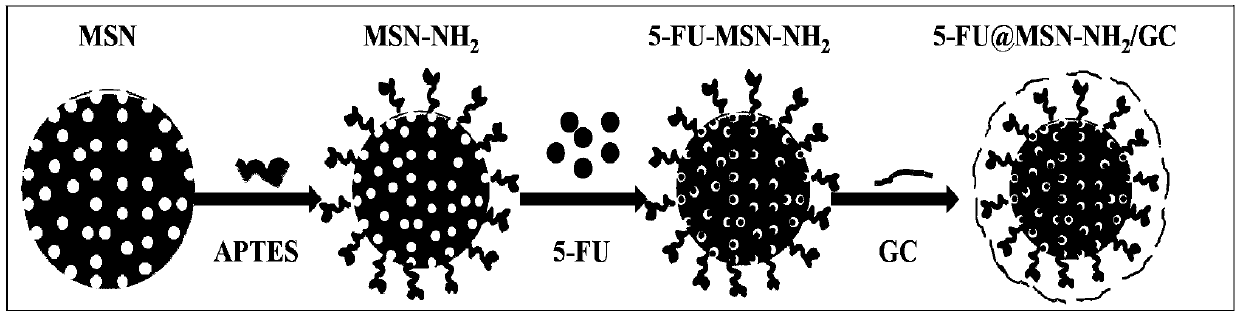
[0060] 1. Synthesis of MSN
[0061] Weigh 1.0g CTAB, add 480mL ultrapure water, stir gently to dissolve CTAB, adjust the oil bath temperature to 80°C, add 3.5mL 2M NaOH solution, stir for 20min, then add 5.0mLTEOS solution dropwise to the mixture, continue After two hours of reaction, stop the reaction and let it cool down, centrifuge (12000rpm, 5min), wash with ultrapure water and absolute ethanol three times, and dry in a vacuum oven at 50°C for 12h to obtain crude MSN. Add 50mL of absolute ethanol and 0.5mL of HCl (37.2%) for every 0.5g of crude MSN, the oil bath temperature is 79°C, reflux for 6h, centrifuge (12000rpm, 5min), discard the supernatant, wash with ultrapure water and absolute ethanol Three times, placed in a drying oven to dry for 12 hours, to obtain MSN without template.
[0062] 2. MSN-NH 2 Synthesis
[0063] The prepared MSNs were dried at 140 °C for 1 h. Add 1.0g of MSN to 7mL of APTES, stir, mix evenly, add to a round bottom flask filled with 50mL of ...
Embodiment 2
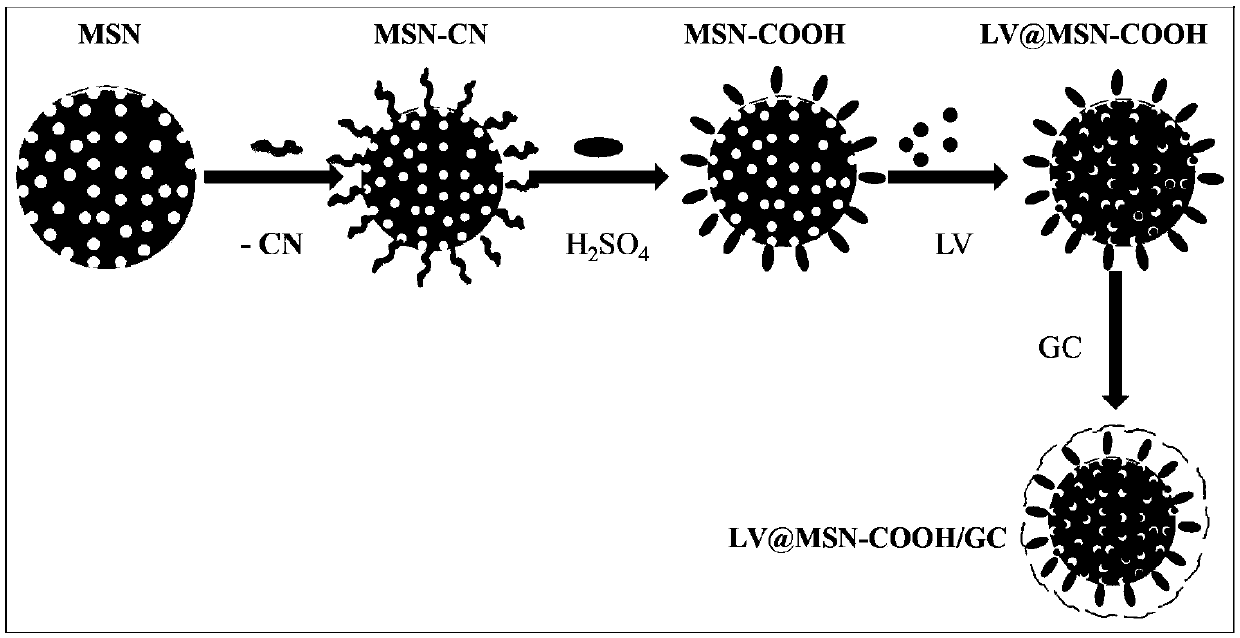
[0072] 1. Synthesis of MSN-COOH
[0073] Weigh 1.2g CTAB and add it to a 500mL round bottom flask containing a mixture of 180mL and 5.5mL ammonia (25%), then vigorously stir in a water bath at 60°C for 30min, then quickly add 2.0mL TEOS and 0.4mL 2- Cyanoethyltriethoxysilane, stirred for another 2h; stood still at the same temperature for 24h, and centrifuged (20000rpm, 20min). Then re-disperse twice with deionized water and ethanol respectively, and dry at 50°C. Add the product to 30mL of 9mol / L sulfuric acid after drying, and react in an oil bath at 100°C for 18h. After the reaction, centrifuge (20000rpm, 20min), and continue to treat the product with 9% hydrochloric acid ethanol solution at 65°C for 24h, and centrifuge (20000rpm, 20min), dry overnight, which is MSN-COOH.
[0074] 2. Synthesis of MSN-COOH / GC
[0075] Measure 5mL of PBS buffered saline solution with pH 7.4 into a 25mL round bottom flask, then add 5mL of GC solution (5mg / mL), and stir well. Then 30 mg of d...
Embodiment 3
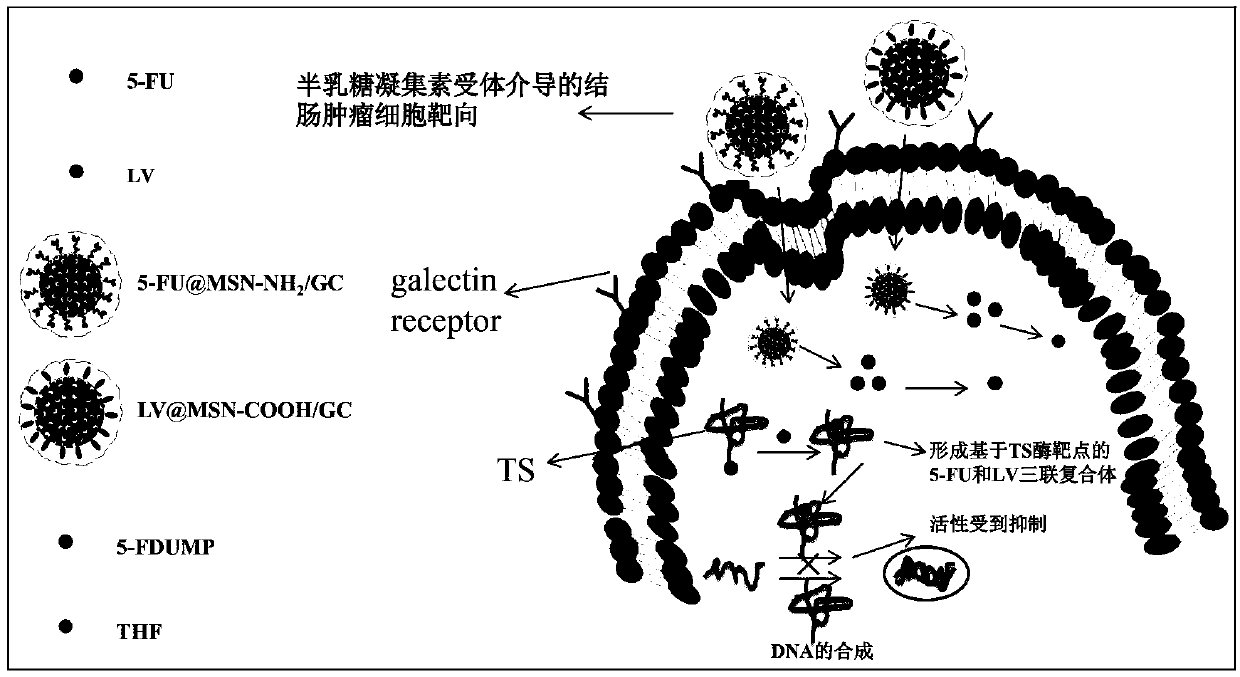
[0082] Take SW620 in the logarithmic phase, trypsinize and collect, inoculate in a 12-well plate (3×104 cells / well) and incubate for 24 hours, discard the original culture medium, add fluorescein isothiocyanate (FITC), FITC@ MSN-NH 2 , FITC@MSN-NH 2 / GC culture solution, the concentration is 50μg / mL. After incubation for 4 hours, the culture medium was discarded, washed three times with PBS, and the uptake of samples by SW620 cells was analyzed with a fluorescence microscope. To assess the competitive effect of galactose on cellular uptake, set FITC@MSN-NH 2 A group of / GC+galactose, that is, in FITC@MSN-NH 2 Add galactose solution with a concentration of 2mg / ml 30min before GC application; suck out the galactose-containing medium after incubation for 30min, and add FITC@MSN-NH 2 / GC (50μg / ml) was added to the wells, and the results of fluorescence microscope analysis were compared with those of MSN-NH without galactose 2 / GC for comparative analysis.
[0083] From Fig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com