Bifunctional water electrolysis catalyst and preparation method and application thereof
A water electrolysis and dual-function technology, applied in chemical instruments and methods, physical/chemical process catalysts, electrolytic components, etc., can solve the problems of limited reports and achieve the effect of rich morphology, simple and convenient operation, and excellent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] NeS 2 The growth of the nanotube material was accomplished as follows: 0.2 mmol nickel chloride (NiCl 2 ·6H 2 O), 0.5 mmol sodium thiosulfate (Na 2 S 2 o 3 ·6H 2 O) and 0.11g of polyvinylpyrrolidone were dissolved in 60mL of deionized water to obtain an aqueous reaction solution; the aqueous reaction solution was transferred to a 100mL polytetrafluoroethylene autoclave, and the treated foamed nickel (size 1 × 3cm) was put into the reaction in the cauldron. Keep the reactor at 150°C for 10h, after cooling to room temperature, wash the nickel foam thoroughly with deionized water and ethanol, and then dry it at 70°C for 10h to obtain a bifunctional electrolytic water catalyst.
Embodiment 2
[0029] NeS 2 The growth of the nanotube material was accomplished as follows: 0.2 mmol nickel chloride (NiCl 2 ·6H 2 O), 0.5 mmol sodium thiosulfate (Na 2 S 2 o 3 ·6H 2 O) and 0.11g of polyvinylpyrrolidone were dissolved in 70mL of deionized water to obtain a reaction aqueous solution; the reaction solution was transferred to a 100mL polytetrafluoroethylene autoclave, and the treated foamed nickel (size 1 × 3cm) was put into the reaction in the cauldron. Keep the reactor at 155°C for 10h, after cooling to room temperature, wash the nickel foam thoroughly with deionized water and ethanol, and then dry it at 70°C for 10h to obtain a bifunctional electrolytic water catalyst.
Embodiment 3
[0031] NeS 2 The growth of the nanotube material was accomplished as follows: 0.2 mmol nickel chloride (NiCl 2 ·6H 2 O), 0.5 mmol sodium thiosulfate (Na 2 S 2 o 3 ·6H 2 O) and 0.11g of polyvinylpyrrolidone were dissolved in 76mL of deionized water to obtain an aqueous reaction solution; the aqueous reaction solution was transferred to a 100mL polytetrafluoroethylene autoclave, and the treated foamed nickel (size 1 × 3cm) was put into the reaction in the cauldron. Keep the reactor at 150°C for 10h, after cooling to room temperature, wash the nickel foam thoroughly with deionized water and ethanol, and then dry it at 70°C for 10h to obtain a bifunctional electrolytic water catalyst.
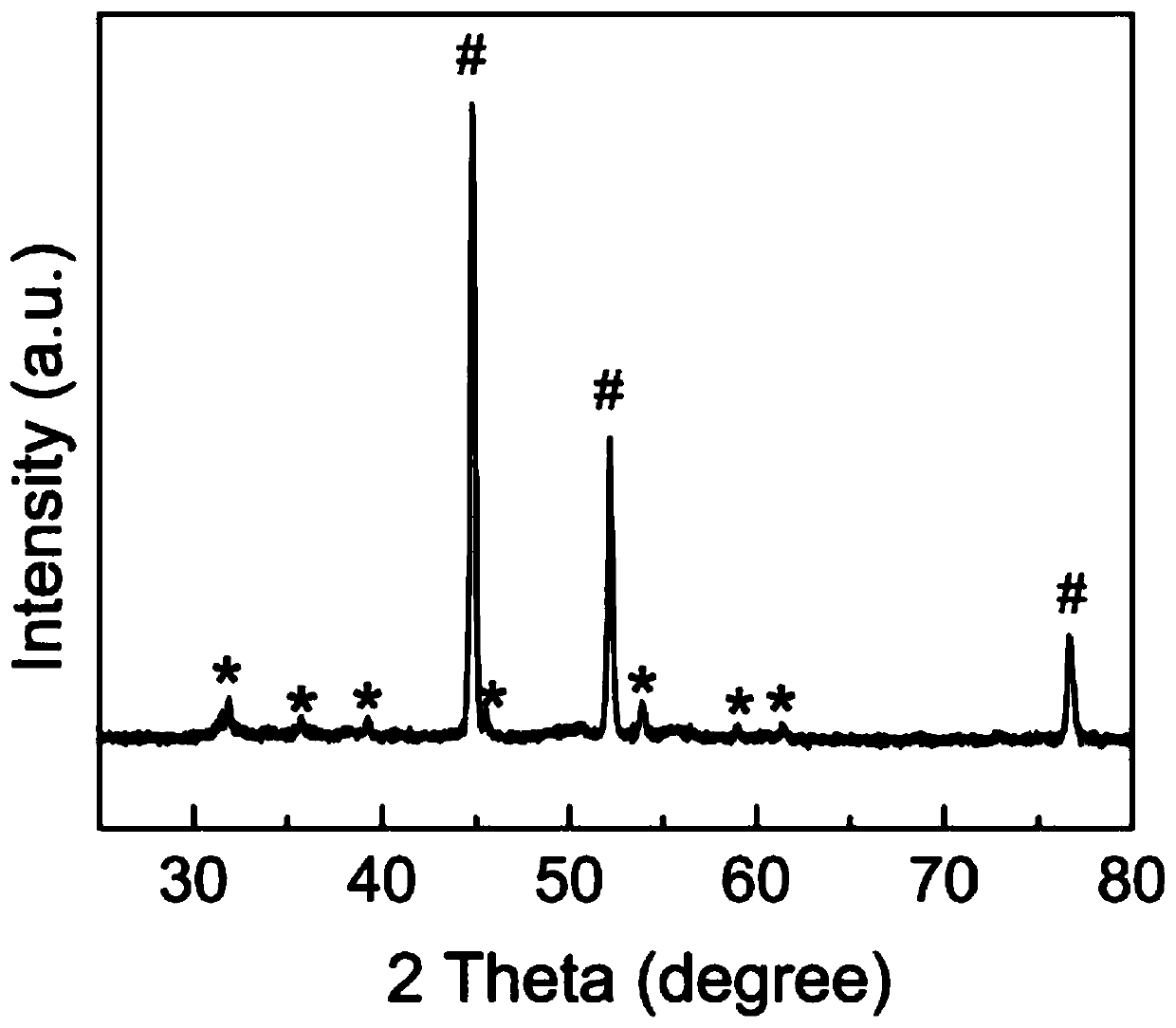
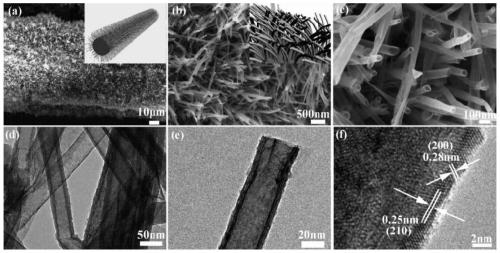
[0032] In the prepared bifunctional electrolytic water catalyst, NiS 2 The nanotubes grow approximately vertically on the nickel foam substrate in the form of arrays, with a diameter of 20-80nm and a length of 100-500nm.
[0033]The process of using the bifunctional electrolytic water catal...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com