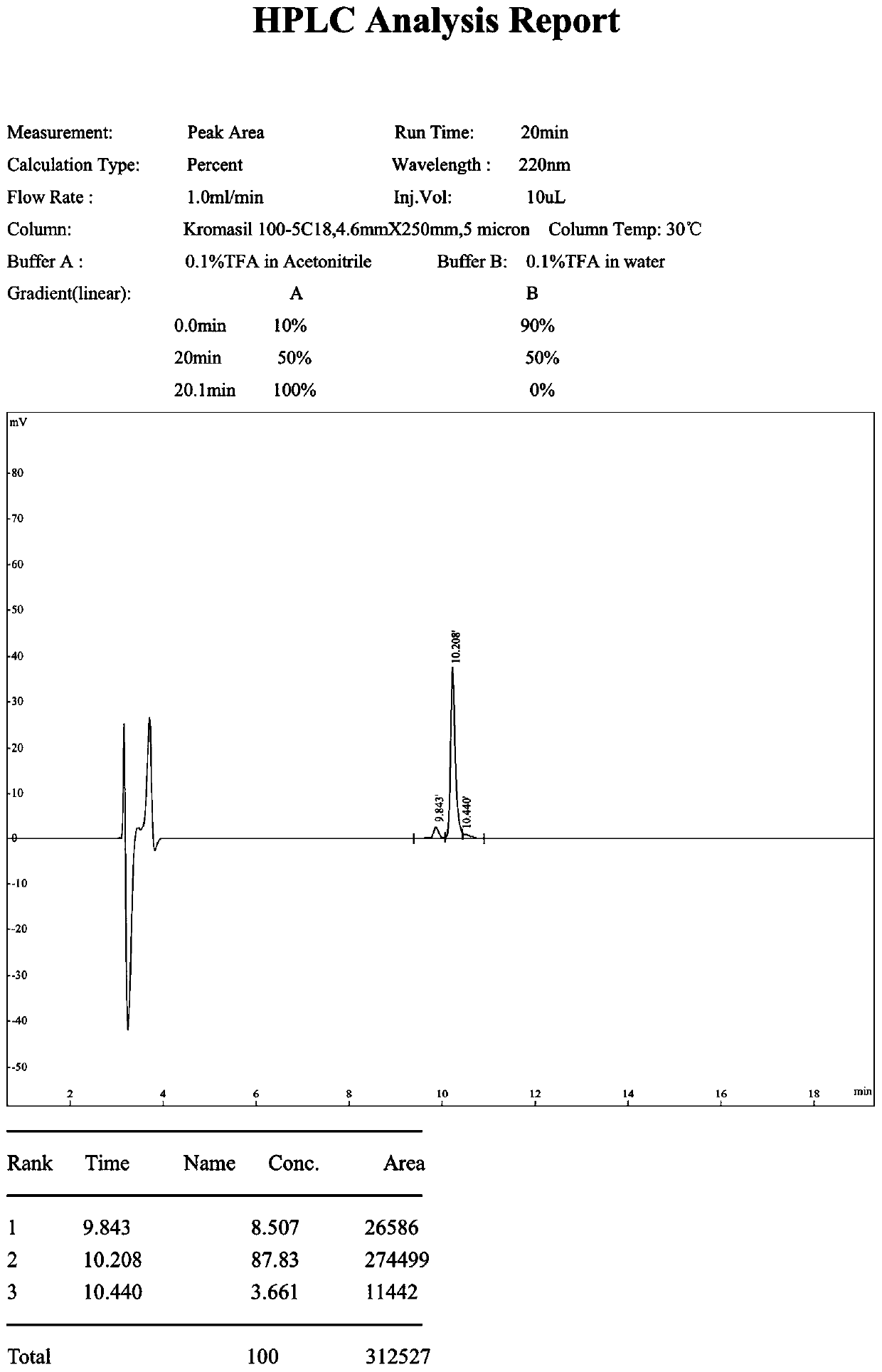
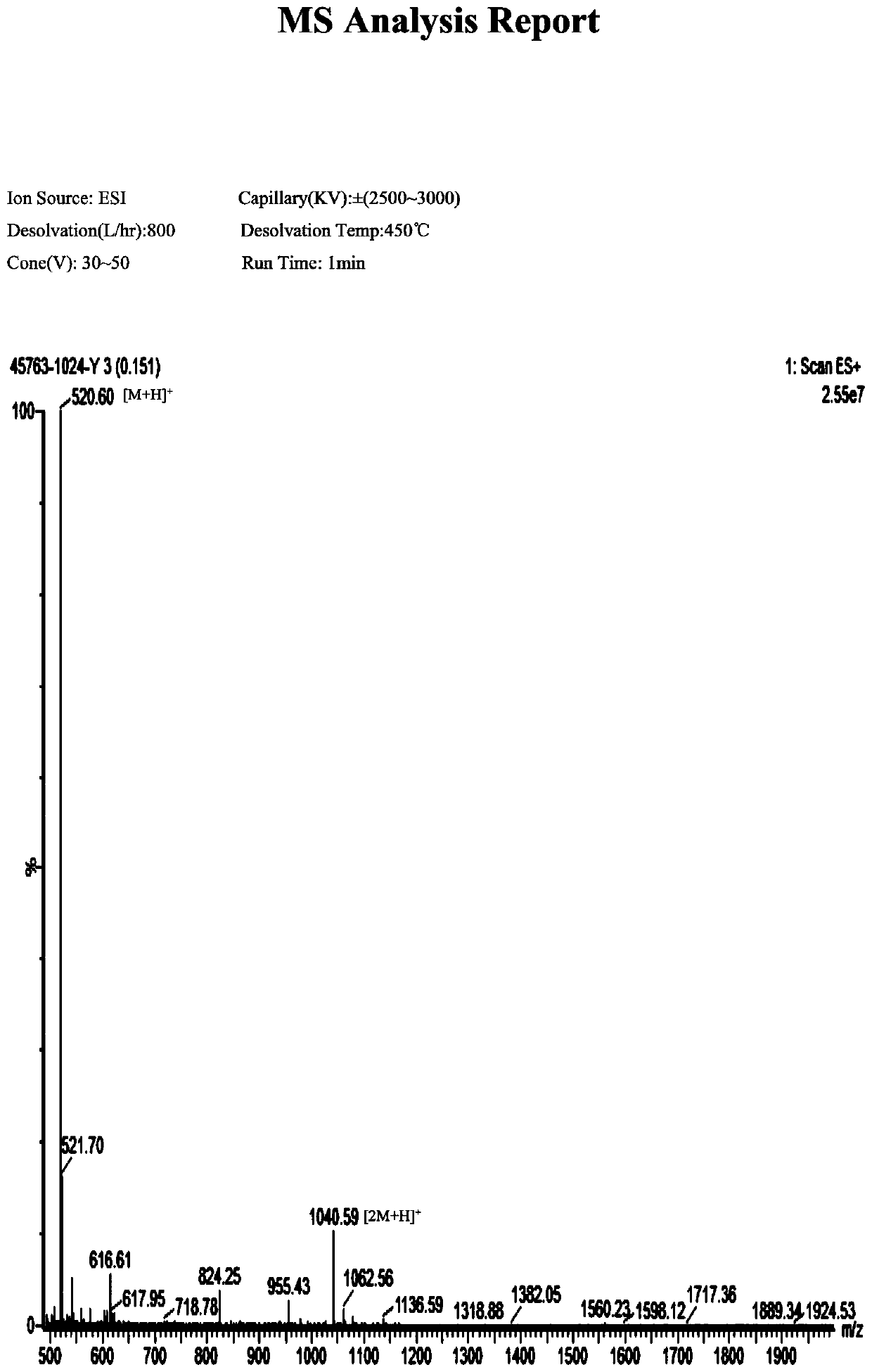
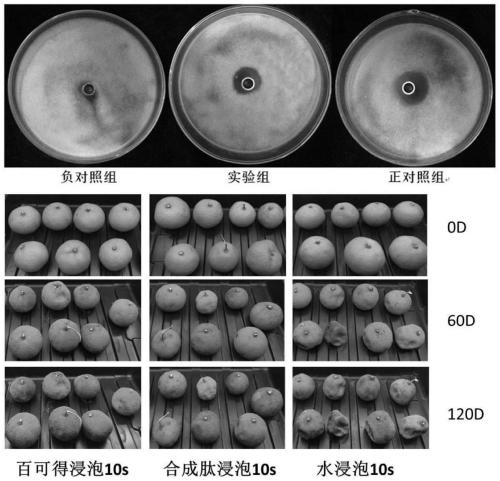
Synthesis of oligopeptide and application of oligopeptide in medicine for inhibiting citrus rot-causing bacteria penicillium digitatum
A technology of oligopeptide and composition, which is applied in the potential fields of citrus preservation and biological control, can solve the problems of cumbersome purification of natural active peptides and small content, and achieve good inhibitory effect, good activity and obvious advantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Solid-phase synthesis process of novel synthetic peptides (from C-terminus to N-terminus):
[0027] (1) The resin is swollen, put it into a 2-chlorotriacyl chloride resin reaction tube, and shake with DCM (15 ml / g) for 30 min.
[0028] (2) Connect the first amino acid, filter out the solvent through sand core, add 3 times molar excess of Fmoc-Ser-OH amino acid relative to the resin, add DMF to dissolve, then add 10 times molar excess DIEA, shake for 60 min, Block with methanol.
[0029] (3) Deprotection, remove DMF, add 20% piperidine DMF solution (15 ml / g) for 5 min, remove and add 20% piperidine DMF solution (15 ml / g), react for 15 min.
[0030] (4) Detection, remove the piperidine solution, take 20 resins, wash with ethanol three times, add ninhydrin reagent for detection, heat at 105 ℃-110 ℃ for 5 min, turn dark blue as a positive reaction, and the deprotection reaction is complete.
[0031] (5) Rinse, followed by DMF (10 ml / g) twice, DCM (10 ml / g) twice, DMF (10 ...
Embodiment 2
[0045] The antibacterial activity of synthetic peptides was measured by the Oxford cup method:
[0046] Prepare sterile water and a clean test tube (sterilized by autoclaving at 121 °C for 20 min), add 10 ml of sterile water to the test tube, and pick out the indicator bacteria that grow well on the plate (ie Penicillium digitorum) Put it into sterile water, shake it thoroughly, filter out the mycelium with sterile cotton, count the number of spores under a microscope, and prepare the bacterial suspension with distilled water to a concentration of 10 6 cfu / mL, ready for use.
[0047] Heat and melt the PDA solid medium (1000 ml of water, 200 g of potato cubes, 20 g of glucose, and 20 g of agar; after autoclaving at 121 °C for 20 min, and then cooling for use), prepare a plate. After the plate is fully solidified, there is water vapor on the surface of the plate After drying, add 400 µl of the spare Penicillium digitorum spore suspension on the surface of the plate, spread it e...
Embodiment 3
[0050] The synthetic peptide structure provided by the present invention is: Abu-N-Me-Thr-Thr-N-Me-Val-Ser, wherein Abu is 2-aminobutyric acid; the comparative oligopeptide structure is: Baba-N-Me-Thr- Thr-N-Me-Val-Ser, in which Baba is 3-aminobutyric acid, the structures of the two oligopeptides are similar, and the only difference is in the connection position of the amino group in the first amino acid on the left, but the activity of the two oligopeptides There are distinct differences.
[0051] Prepare sterile water and a clean test tube, add 10 ml of sterile water to the test tube, pick out the indicator bacteria that grow well on the plate (that is, Penicillium finger) into the sterile water, shake well, and use sterile The mycelium was filtered out of cotton for later use, the number of spores was counted under a microscope, and the bacterial suspension was prepared with distilled water to a concentration of: 10 6 cfu / mL, ready for use. The PDA solid medium was autoc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com