PSD-95 inhibitor
A technology of -AA2 and -AA3 is applied in the direction of peptide-nucleic acid, medical preparations containing active ingredients, peptide/protein components, etc., which can solve the problems of lack of treatment methods and achieve stable properties and significant protective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1 compound 1
[0032] Tyr-Gly-Arg-Lys-Lys-Arg-Arg-Gln-Arg-Arg-Arg-Lys-Leu-Ser-Ser-Ile-
[0033] Glu-Ser-Asp-Val-Lys(Pal-γGlu)-NH 2
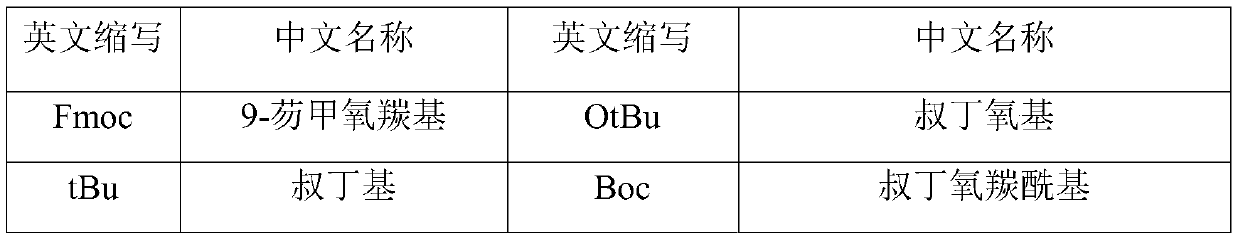
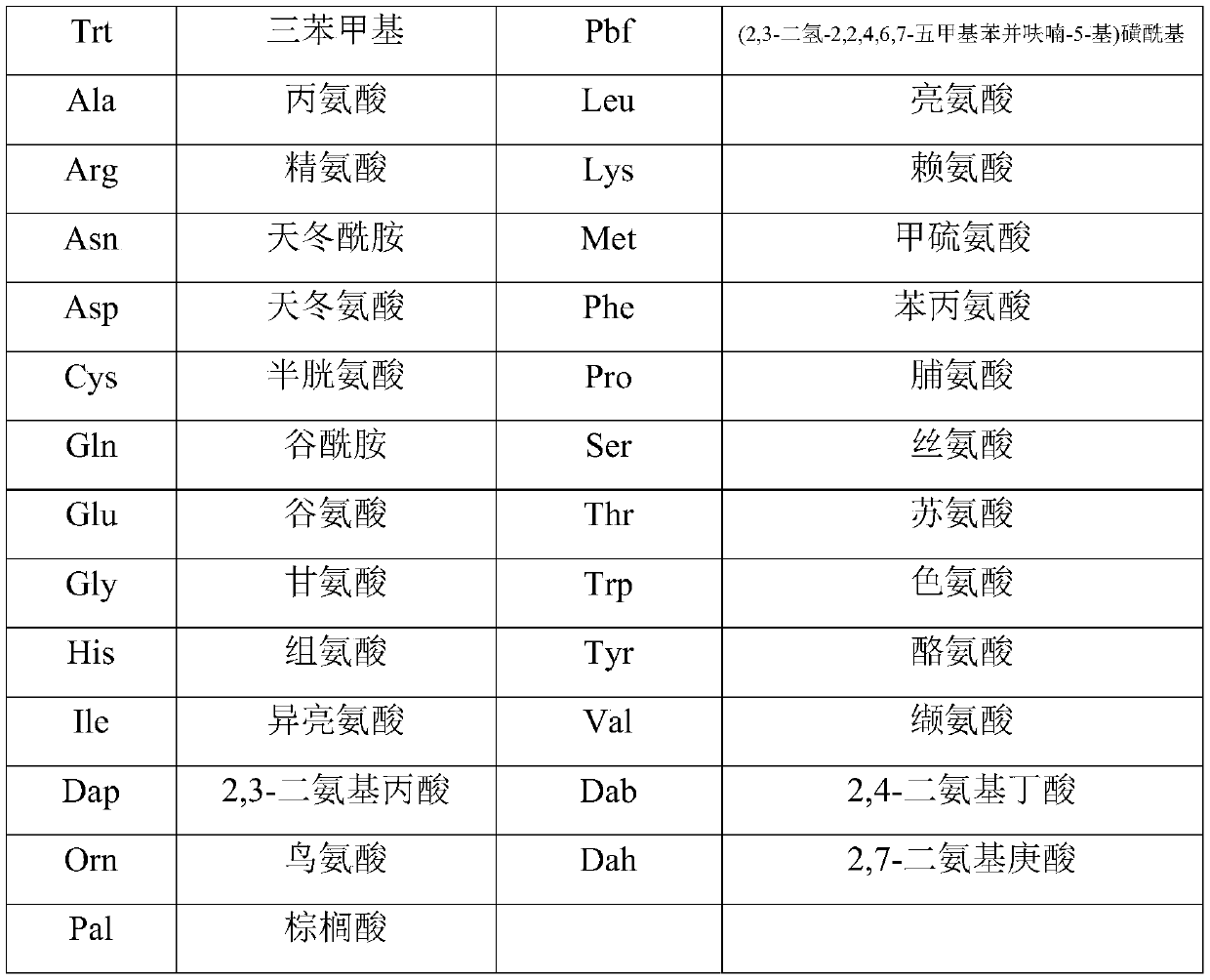
[0034] The preparation method includes: preparing the peptide resin by solid-phase polypeptide synthesis method, acid hydrolyzing the peptide resin to obtain a crude product, and finally purifying the crude product to obtain a pure product; wherein the step of preparing the peptide resin by the solid-phase polypeptide synthesis method is to pass solid-phase The phase-coupled synthesis method sequentially inserts the corresponding protected amino acids or fragments in the following sequences to prepare peptide resins:
[0035] In the above preparation method, the amount of the Fmoc-protected amino acid or protected amino acid fragment is 1.2-6 times of the total moles of the resin fed; preferably 2.5-3.5 times.
[0036] In the above preparation method, the substitution value of the carrier resin is 0.2-1.0 m...
Embodiment 2
[0072] The preparation of embodiment 2 compound 2
[0073] Tyr-Gly-Arg-Lys-Lys-Arg-Arg-Gln-Arg-Arg-Arg-Lys-Leu-Ser-Ser-Ile-
[0074] Glu-Ser-Asp-Val-Lys(2-cholesterol butyrate)-NH 2
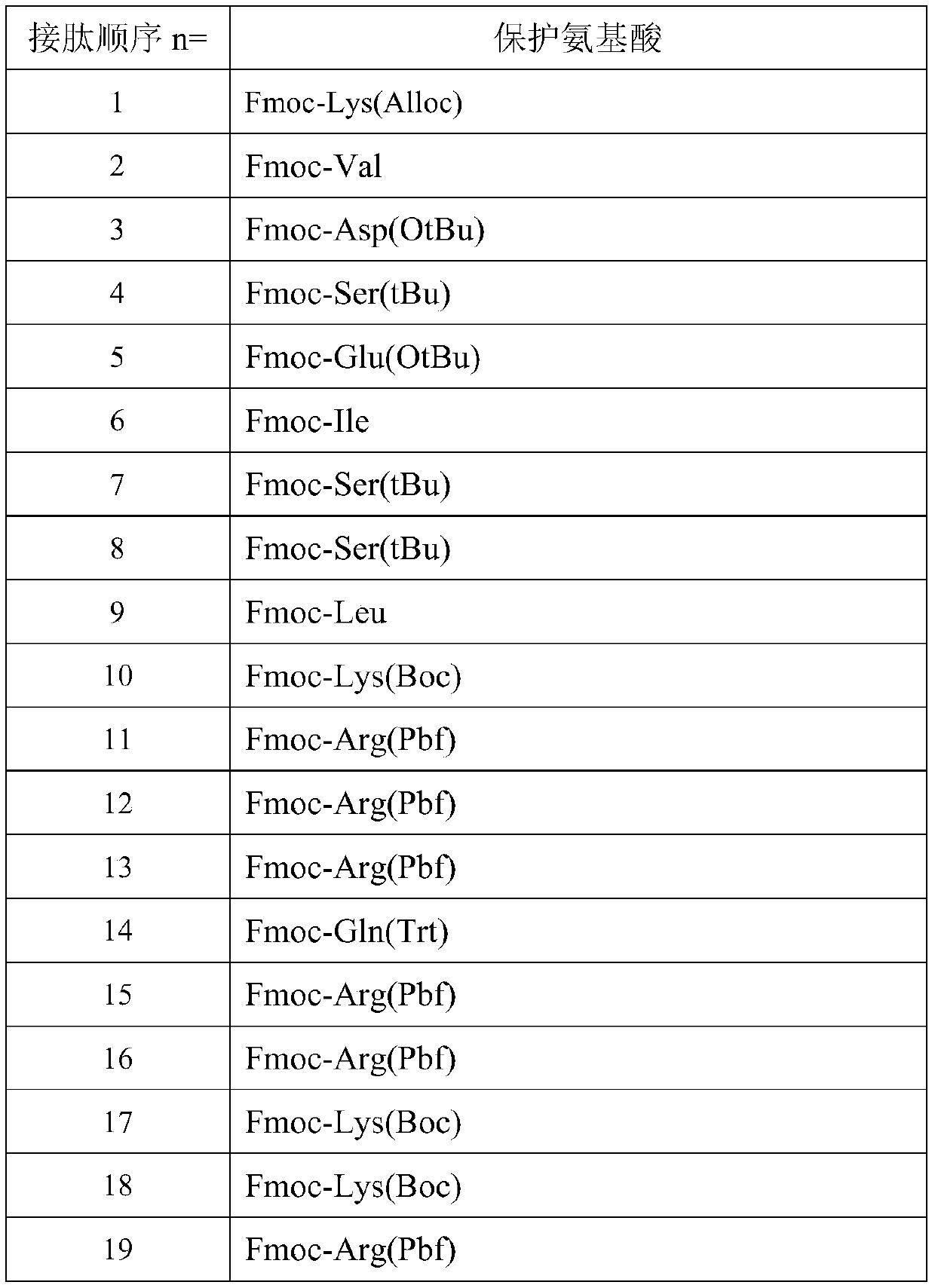
[0075] The preparation method is the same as in Example 1, and the protected amino acids used are as follows:
[0076]
[0077]
[0078] 7.2 g of pure product was obtained, the purity was 95.8%, and the total yield was 23.2%. The molecular weight is 3100.8 (100% M+H).
Embodiment 3
[0079] The preparation of embodiment 3 compound 3
[0080] Tyr-Gly-Arg-Lys-Lys-Arg-Arg-Gln-Arg-Arg-Arg-Lys-Leu-Ser-Ser-Ile-
[0081] Glu-Ser-Asp-Val-Cys(cholesteryl acetate)-NH 2
[0082] 1. Preparation of peptide resin
[0083] Method is the same as embodiment 1, and the protected amino acid that uses is as following table:
[0084]
[0085]
[0086] 2. Preparation of crude product
[0087] Take the above peptide resin, add a cleavage reagent with a volume ratio of TFA: water: EDT = 95: 5: 5 (cleavage reagent 10mL / g resin), stir well, stir and react at room temperature for 3 hours, use a sand core funnel to filter the reaction mixture, collect The filtrate and the resin were washed 3 times with a small amount of TFA, the combined filtrates were concentrated under reduced pressure, anhydrous ether was added to precipitate, and then the precipitate was washed with anhydrous ether 3 times, dried to obtain off-white powder, dissolved in pure DMSO, added Equal molar tri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com