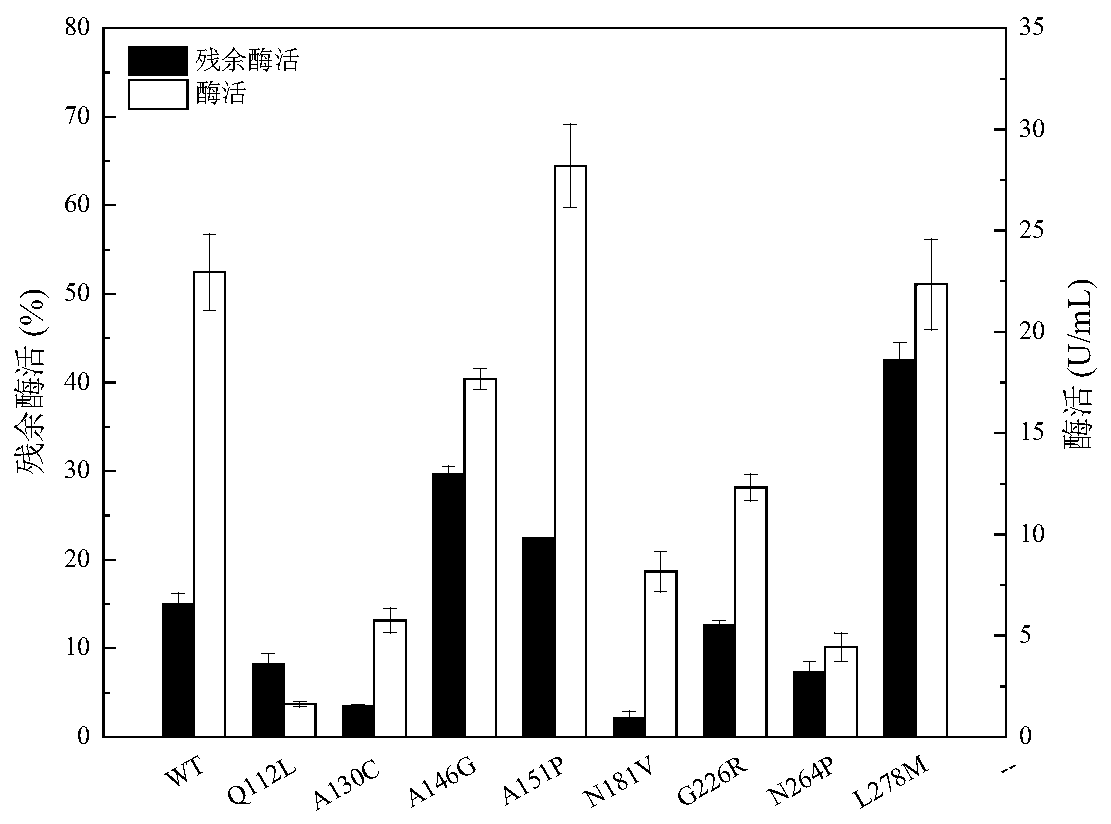
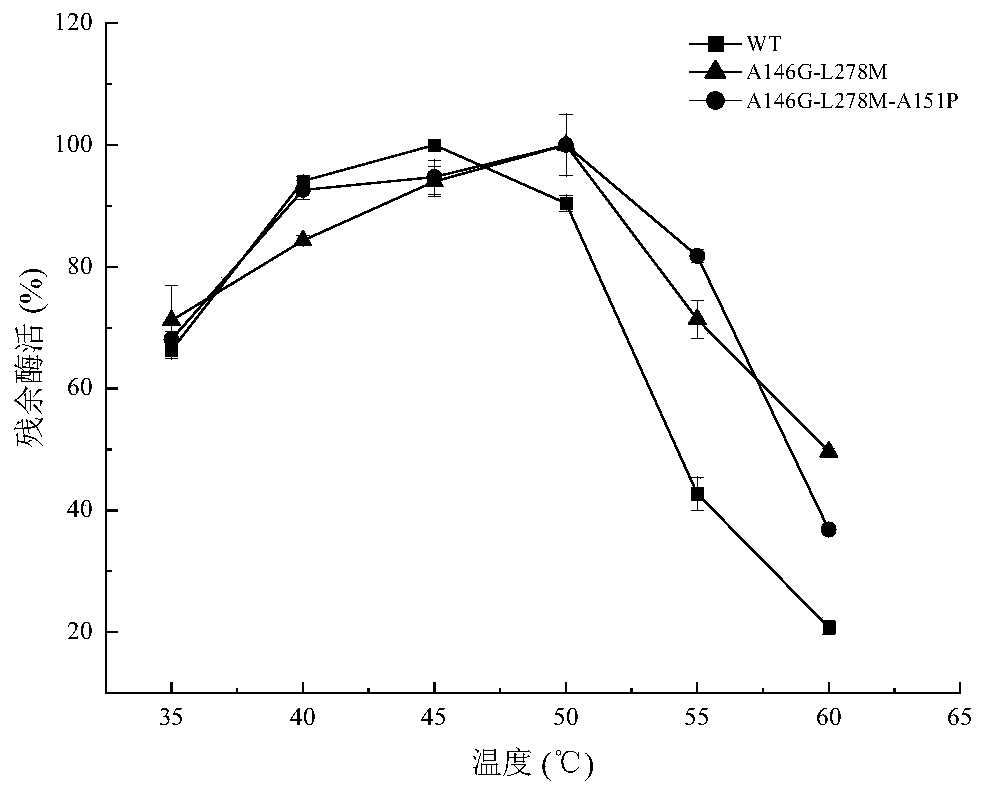
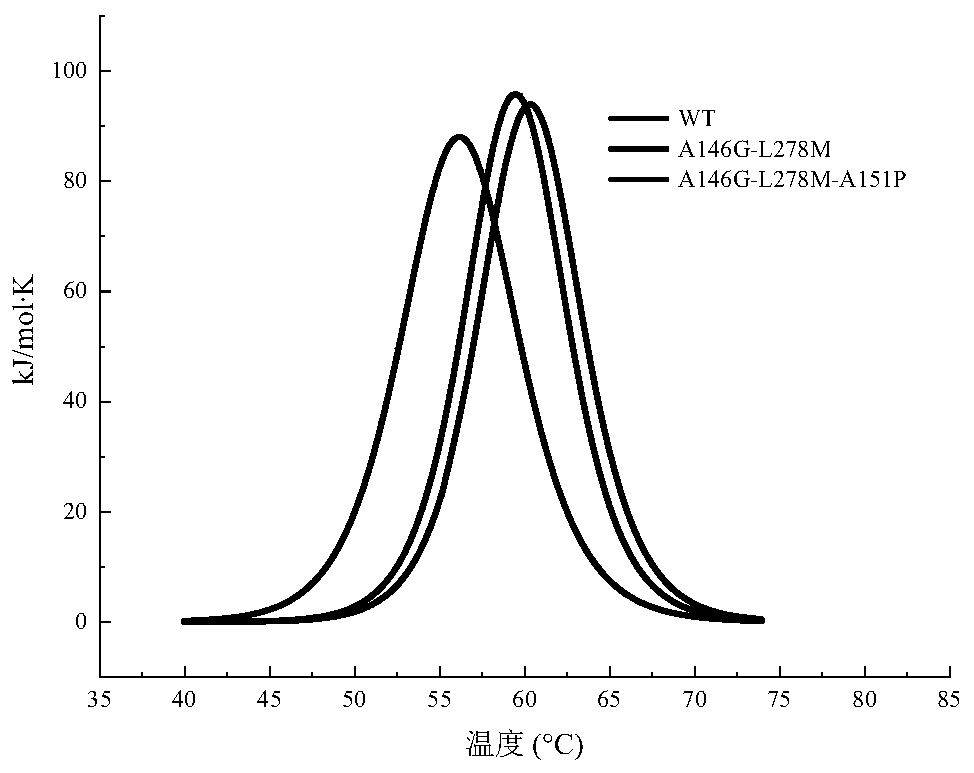
Lipase with improved catalytic performance and application thereof
A technology of lipase and parent enzyme, applied in the field of genetic engineering, to achieve the effects of good thermal stability and catalytic activity, improved catalytic activity, and improved optimal reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The prediction of embodiment 1 lipase mutant mutation site
[0048] Based on the crystal structure of Candida antarctica lipase B, computer-aided protein design was used, and the identified mutation sites are shown in Table 1.
[0049] Table 1 Selection of Mutant Amino Acids
[0050]
Embodiment 2
[0051] Construction of the recombinant plasmid of embodiment 2 lipase mutant
[0052] Carry out primer synthesis according to mutation site, take the gene (nucleotide sequence as shown in SEQ ID NO.1) of the lipase B of coding Candida antarctica origin as template, carry out site-directed mutagenesis by overlap extension PCR (site-directed mutagenesis primer see Table 2). After the obtained point mutant gene was amplified by PCR, the point mutant gene was connected to the expression vector pPICZαA by T4 ligase. Subsequently, the ligation product was transformed into Escherichia coli JM109 competent by chemical transformation method, and spread to contain 25 μg·mL -1 Bleomycin LLB Screening Plate. The transformants were picked to extract plasmids, sequenced and identified, and finally recombinant plasmids pPICZαA-A130C, pPICZαA-A146G, pPICZαA-N181V, pPICZαA-N264P, pPICZαA-L278M, pPICZαA-S50R, pPICZαA-S56M, pPICZαA- Q112L, pPICZαA-A151P, pPICZαA-G226R.
[0053] Table 2 Prime...
Embodiment 3
[0056] Transformation and verification of the recombinant plasmid of embodiment 3 lipase mutant
[0057] The above-mentioned recombinant plasmids were linearized respectively using restriction endonuclease PmeI. Take 200ng of linearized fragments respectively, add them to Pichia pastoris GS115 competent cells, mix well, transfer to the electroporation cup, and use electroporation (Eppendorf) for 5 minutes after electroporation, add 1mL sorbitol for recovery after electroporation, and incubate at 30℃ , 200rpm under recovery culture 2h. Then the resuscitation solution was spread to contain 100 μg·mL -1 Bleomycin YPD screening plate, cultivated for 2-3 days.
[0058]Transformants were picked and inoculated into YPD medium, and cultured overnight. Collect the bacteria to extract the genome. Using the genome as a template, the universal primers 3'AOX and 5'AOX were used for PCR verification. Positive clones can be amplified to obtain a 1500bp band.
[0059] The general primer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com