Preparation method of 3, 5-bis (trifluoromethyl) acetophenone
A technology of trifluoromethyl acetophenone and trifluoromethyl bromide, which is applied in the field of organic synthesis and pharmaceuticals, can solve the problems of high cost, long reaction steps, complicated steps, etc., and achieve good social and economic benefits and economic value The effect of high potential and simple reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] Its preparation method comprises the following steps:
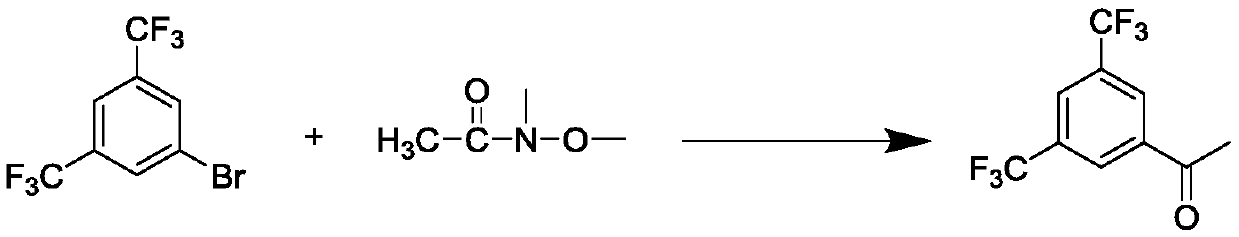
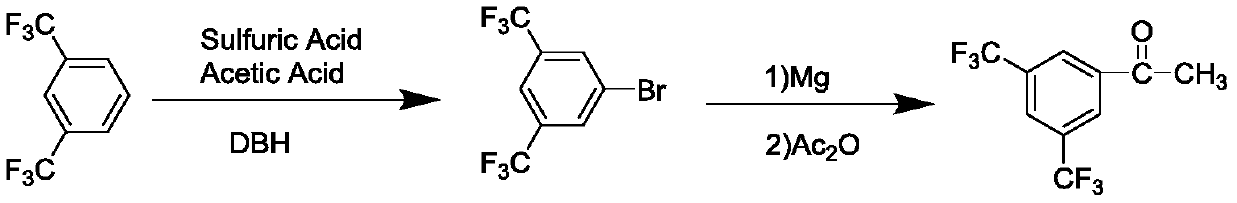
[0031] Q1, the reaction step, at the reaction temperature, select N-methoxy-N-methylacetamide or N-methoxy-N-methylformamide and 3,5-bistrifluoromethyl bromobenzene, n- Mix butyllithium, stir and react under the condition of reaction solvent, the reaction temperature is -70~-90°C;
[0032] Q2, the post-processing step, raised to room temperature, extracted, washed, dried, and evaporated to remove the residual solvent, separated by column chromatography to obtain 3,5-bistrifluoromethylacetophenone.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com