Expression method of earthworm antibacterial peptide
An expression method and antibacterial peptide technology, applied in the fields of biotechnology and genetic engineering, can solve the problems of difficult purification, easy inhibition, harm, etc., and achieve the effect of simple purification work
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
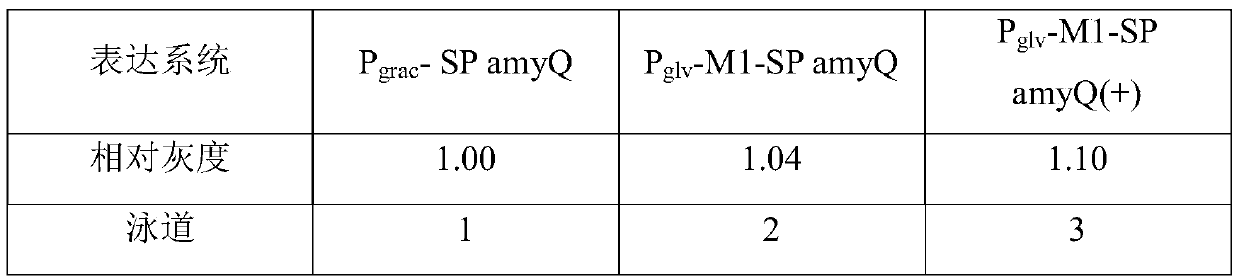
[0034] Example 1, P glv High expression of -M1-SP amyQ(+)-SUMO-Lumbricin I(6-34) in Bacillus subtilis Efficient expression
[0035] A) Optimization of the Bacillus subtilis WB800N genome
[0036] The P43 promoter sequence in the Bacillus subtilis WB800N genome, the front-end sequence GAF and the back-end sequence GAB of the Pglv promoter, and the kanamycin resistance gene KAN in the plasmid were respectively amplified by PCR.
[0037] Synthesize the following primers respectively:
[0038] F(GAF):GTTTCCTGACACACCGTTCACC (SEQ ID NO:6)
[0039] R(GAF): GTAGTTAGGCCACCACTTGAATTTTAAGTGAATATACGCCCTT (SEQ ID NO: 7)
[0040] F(KAN):TCAAGTGGTGGCCTAACTACGGCT (SEQ ID NO:8)
[0041] R(KAN):CCACTCAATAATGCTGAGTTAGAAAAACTCATCGAGCATCAAA (SEQ ID NO:9)
[0042] F(P43):AACTCAGCATTATTGAGTGGATGATTATAT (SEQ ID NO:10)
[0043] R(P43): CTTCATCATTCCTCTCTCTTACCTATAATGGTACCG (SEQ ID NO: 11)
[0044] F(GAB): GTAAGAGAGGAATGATGAAGAAAAAATCATTCTCAATCGT (SEQ ID NO: 12)
[0045] R(GAB): GGAATAATTGAG...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com