Artemisia rupestris L. nasal spray with antiviral activity
An antiviral activity and spray technology, which can be applied to antiviral agents, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problems of low absorption and utilization, and achieve accurate dosage, The effect of improving bioavailability and good spray characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Weigh 0.2g of Artemisia annua extract in each portion, and use different concentrations of ethanol and NaHCO 3 solution, Tween-80 solution and the combined use of mixed solvents, prepare a Artemisia nasal spray solution according to the proportion shown in Table 3 respectively, and observe the drug dissolution situation;
[0037] Table 3 The prescription composition of different solubilization methods of Artemisia nasal spray
[0038]
[0039]
[0040] Continued Table 3
[0041]
[0042] The dissolution observation results of prescriptions 1-14 shown in the above table 3 show that the Artemisia annua extract is dissolved in a certain concentration range of NaHCO 3 It can be completely dissolved in the dissolution of Tween 80, and the solubility of the Artemisia annua extract can also be increased by using a joint method.
Embodiment 2
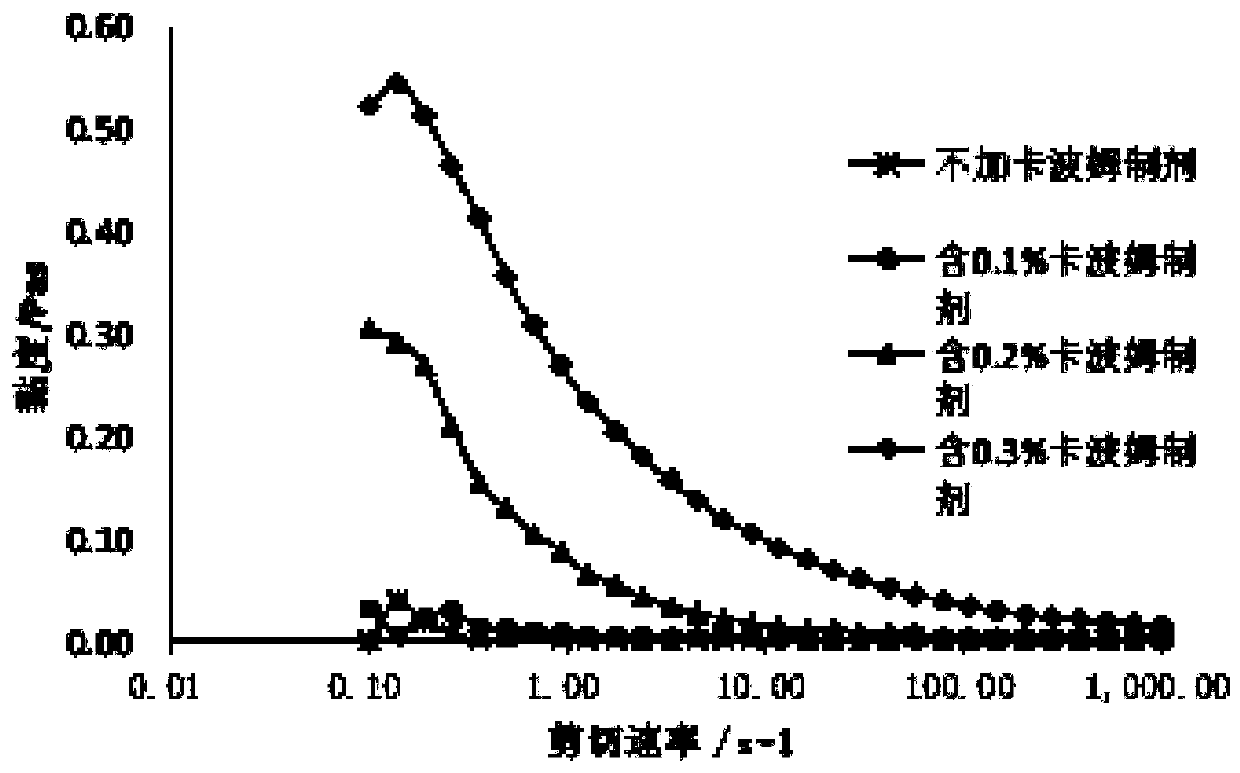
[0044] Prepare 0.1%, 0.2%, 0.3%, 0.5% w / w Carbomer 940 Nasal Spray and 0.15%, 0.2%, 0.25%, 0.5% w / w Carbomer 971P nasal spray, inspect its pH and viscosity, and spray out droplets with a nebulizer, inspect the particle size and adhesion degree, and evaluate the possibility of forming preparations; the results show that each Carbomer 940 preparation is a yellow solution, and the Carbomer 971P preparation is yellow solution. It is a yellow clear solution. 0.3%, 0.5% w / w Carbomer 940 preparations and 0.25%, 0.5% Carbomer 971P preparations have high viscosity and low pH. When the mist is sprayed by the nebulizer, it will be clogged, and the ideal nasal cavity cannot be formed. Preparations: 0.1%, 0.2% Carbomer 940 preparations and 0.15%, 0.2% Carbomer 971P preparations have moderate viscosity, pH is more suitable for nasal administration, and can be sprayed out by sprayer.
Embodiment 3
[0046] According to the prescription shown in Table 4, prepare different models of an Artemisia nasal spray containing poloxamer;
[0047] A Artemisia nasal spray prescription (w / w%) of different adhesive pharmaceutical excipients of table 4
[0048]
[0049] Dissolve the hydrophilic material in the prescription 15-19 in an appropriate amount of deionized water, heat to 100°C under magnetic stirring to disperse evenly, and then place it in a refrigerator at 4°C to completely cool (more than 24h) to obtain a clear solution. In addition, the drug solution and the rest of the excipients were dissolved in deionized water, the two liquids were mixed and dissolved to the full amount, and the temperature sensitivity and spray characteristics of each preparation were investigated. The results showed that with the increase of the amount of poloxamer, the nebulized solution It has certain temperature sensitivity characteristics, but it also affects the size of atomized droplets and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com