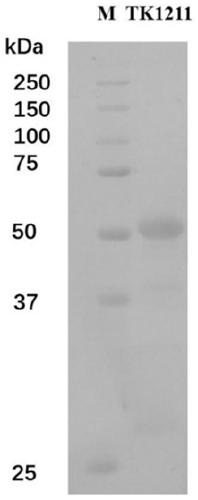
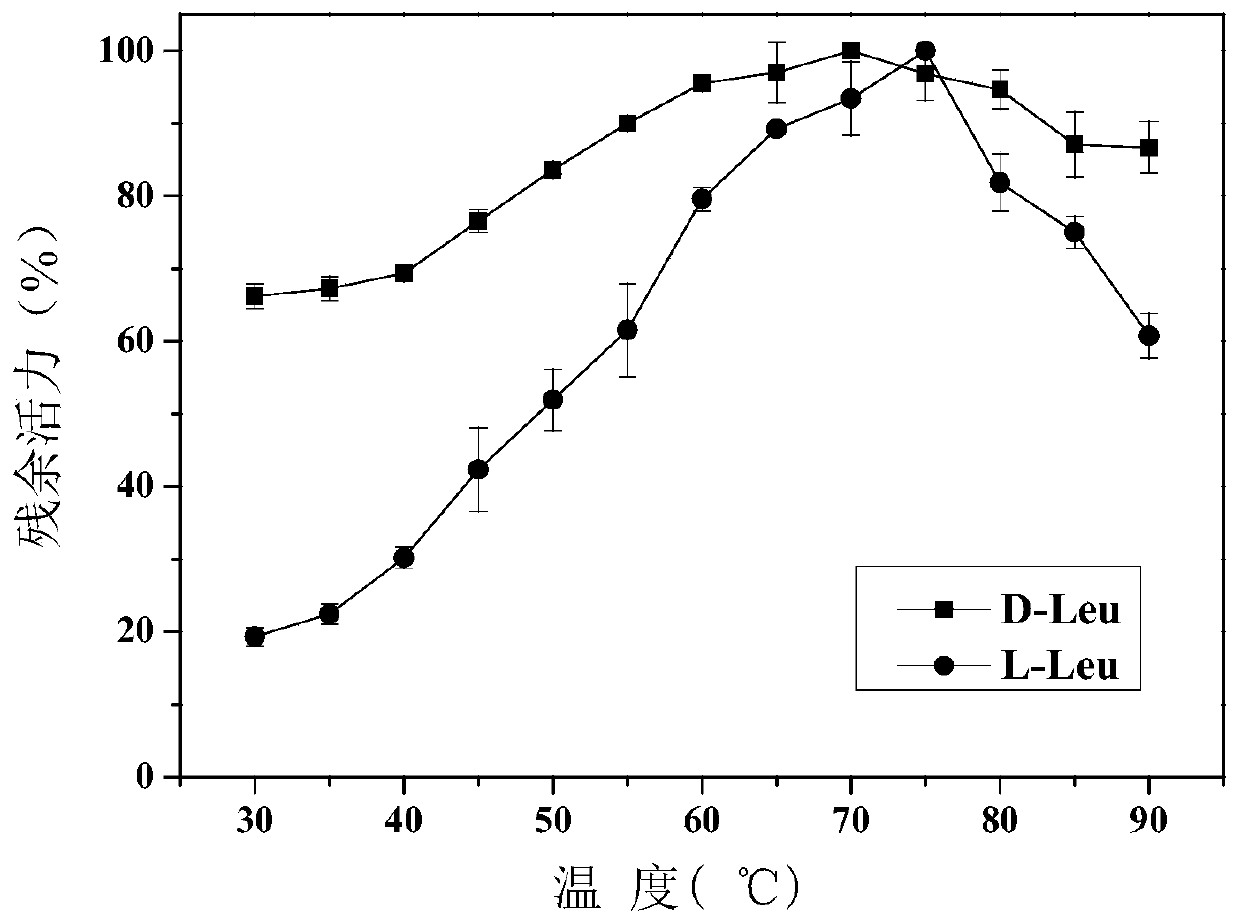
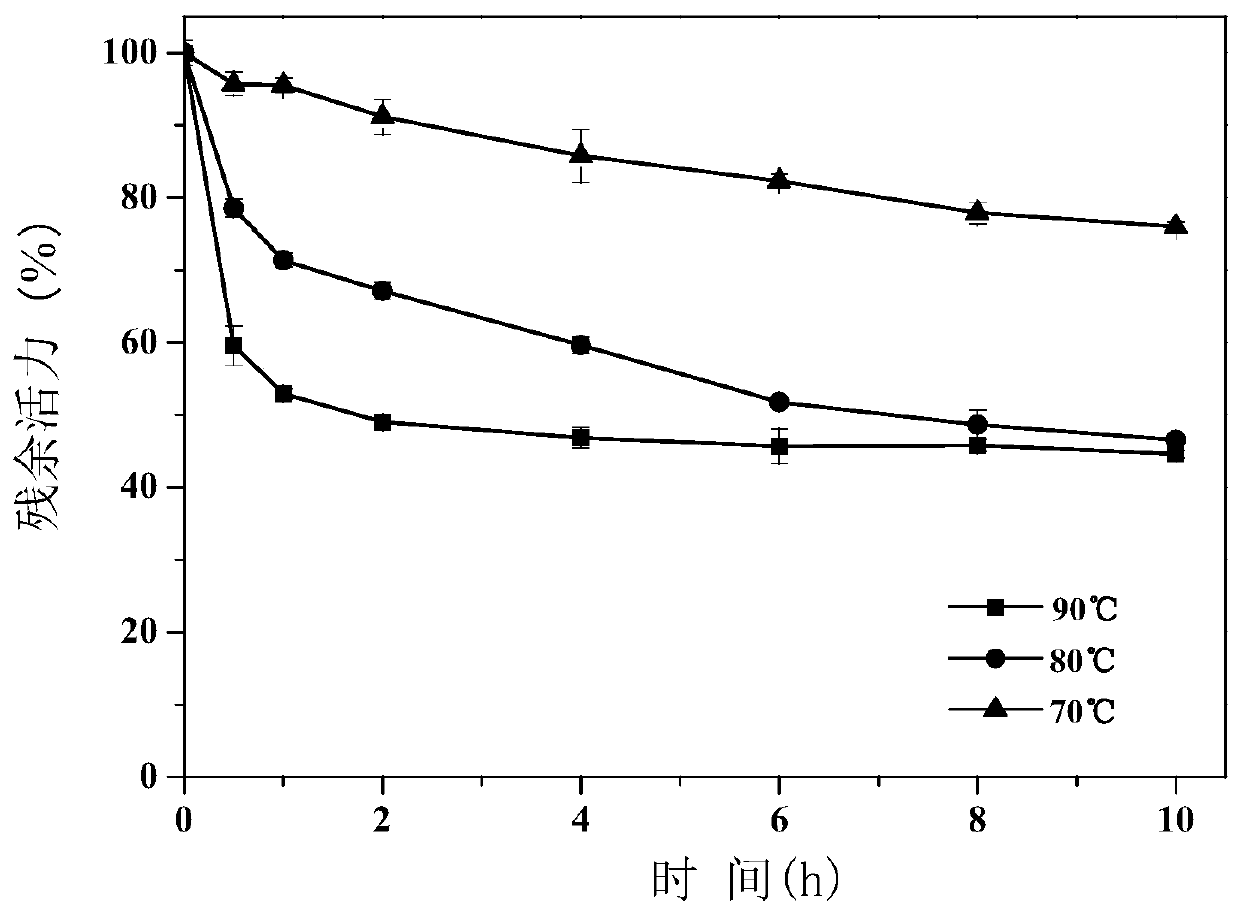
Amino acid racemase and application thereof
An amino acid and leucine technology, applied in the direction of racemase/epimerase, enzyme, isomerase, etc., can solve the problem of narrow substrate spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1. Construction of recombinant expression vector
[0031] The coding gene TkAAR (nucleotide sequence shown in SEQ ID NO. 2) was ligated to the cloning vector pUC118, and the ligation product was transformed into Escherichia coli DH5α competent cells, and spread on LB plate medium containing ampicillin . The single bacteria were picked and then cultured in LB liquid medium and the plasmid was extracted for sequencing. The cloned vector pUC118-TK1211 was the one with correct sequencing result.
[0032] The recombinant cloning vector pUC118-TK1211 and the ESS00988 vector containing the strong promoter Pcsg were treated with double enzyme digestion with NdeI and SalI, and the target gene after double digestion was connected to the vector overnight at 16°C. The ligation product was transformed into E. coli DH5α competent cells, and then plated on LB plate medium containing ampicillin after resuscitation. Pick a single colony, insert it into LB liquid medium for culture, extract...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com