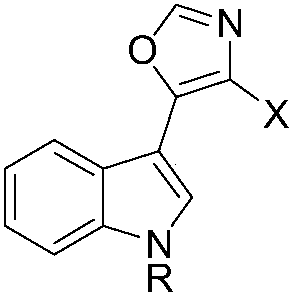
Preparation method and application of natural product Streptochlorin and derivatives thereof
A technology of natural products and derivatives, applied in botany equipment and methods, chemicals for biological control, applications, etc., can solve the problem of less Streptochlorin and its derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Synthesis of Compound 5 (X=Cl, Streptochlorin)
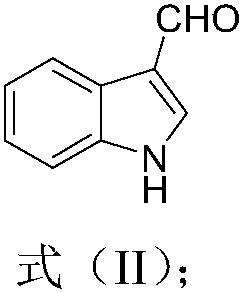
[0065] 1. Synthesis of indole-3-carbaldehyde
[0066] Taking indole (compound 1) as raw material to synthesize raw material indole-3-carboxaldehyde (compound 2), the synthetic route is as follows:
[0067]
[0068] Take a 100mL round bottom flask, add 14.0mL of N,N-dimethylformamide, cool in an ice-water bath to 0-5°C; add POCl with a syringe 3 2.0mL, stirred in an ice-water bath for 30min; Dissolve 2.34g (20.0mmoL) of indole in 5mL of N,N-dimethylformamide, add and react at 35°C for 1h; cool, add H 2 O12.0mL, 26mL of 30% NaOH aqueous solution, heated to reflux for 30min; cooled, a large number of light yellow crystals naturally precipitated; filtered with suction, washed the solid with water several times to remove the alkali and N,N-dimethylformamide in the system; Weigh after drying. The calculated yield is 97%. Mp: 195.8-198.7°C. 1 H NMR (400MHz, DMSO) δ12.14(s, 1H), 9.94(s, 1H), 8.29(s, 1H), 8.10(d, J=7.2Hz, ...
Embodiment 2
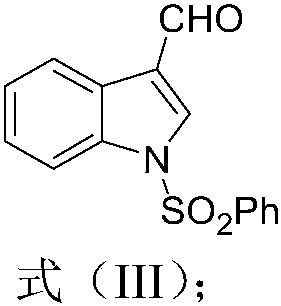
[0082] After completing the framework construction of Streptochlorin according to the synthetic route of Example 1, carry out structural modification to it, the modification site is concentrated on the free NH position of the indole ring, and combine it with different halogenated groups RX in the base of sodium hydride Under neutral conditions, the reaction ranges from 0.25 to 3 hours in the temperature range from room temperature to 100 °C. The obtained compounds were 1 H NMR, 13 Confirmation by C NMR, IR and HRMS.
[0083] 1. Synthesis of compound I-3
[0084] Compound 5 (X=Cl) (0.20mmoL) was obtained by using the synthesis route of Example 1 above. Take 3mL of anhydrous tetrahydrofuran and stir to dissolve; under ice-water bath conditions, add sodium hydride (60%, 0.30mmoL) and react at room temperature for 30min ; Add 2mL of anhydrous tetrahydrofuran containing allyl bromide (0.24mmoL), react at room temperature for 2h, and monitor the progress of the reaction by TLC. ...
Embodiment 3
[0174] The heating conditions in the reaction conditions have been further optimized. Some compounds are heated by microwaves. After screening the reaction temperature, solvent, radiation intensity and other conditions, the microwave reaction only takes 15 minutes to obtain the target compound. The obtained compounds were 1 H NMR, 13 Confirmation by C NMR, IR and HRMS.
[0175] 1. Synthesis of compound I-18:
[0176] After the compound 5 (X=Cl) was obtained by using the synthesis route of the above-mentioned Example 1, the method of microwave-assisted organic synthesis was adopted, and microwave reaction tubes were taken to add microwave magnets, compound 4 (X=Cl) (0.20mmol) and anhydrous Tetrahydrofuran 5mL (dried and re-distilled), after stirring and dissolving, add sodium hydride (60%, 0.40mmol), add a drying tube to react for 20min, add bromomethylcyclopropane (0.24mmol), seal the tube and put it in microwave reaction React in the container for 15 minutes, and the react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com