Carbonic anhydrase fluorescent probe with high brightness and high light stability
A technology of carbonic anhydrase and fluorescent probe, which is applied in the field of fluorescence imaging, can solve the problems of short lifespan and small change in lifespan, and achieve the effects of low price of synthetic raw materials, increased fluorescence lifespan, high brightness and photostability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of carbonic anhydrase probe SML-Aze.
[0031] The synthetic route and product structure of intermediate SML-Br are as follows:
[0032]
[0033] Add 4-bromo-1,8-naphthalene anhydride (2.0 g, 7.2 mmol), 4-(aminomethyl)benzenesulfonamide hydrochloride (3.2 g, 14.4 mmol) and triethylamine (6.8 g, 36mmol), and the reaction solution was heated to 80°C. After 8 hours, the reaction solution was cooled to room temperature and the turbid solution was suction-filtered to obtain 2.94 g of off-white solid SML-Br, with a yield of 92%.
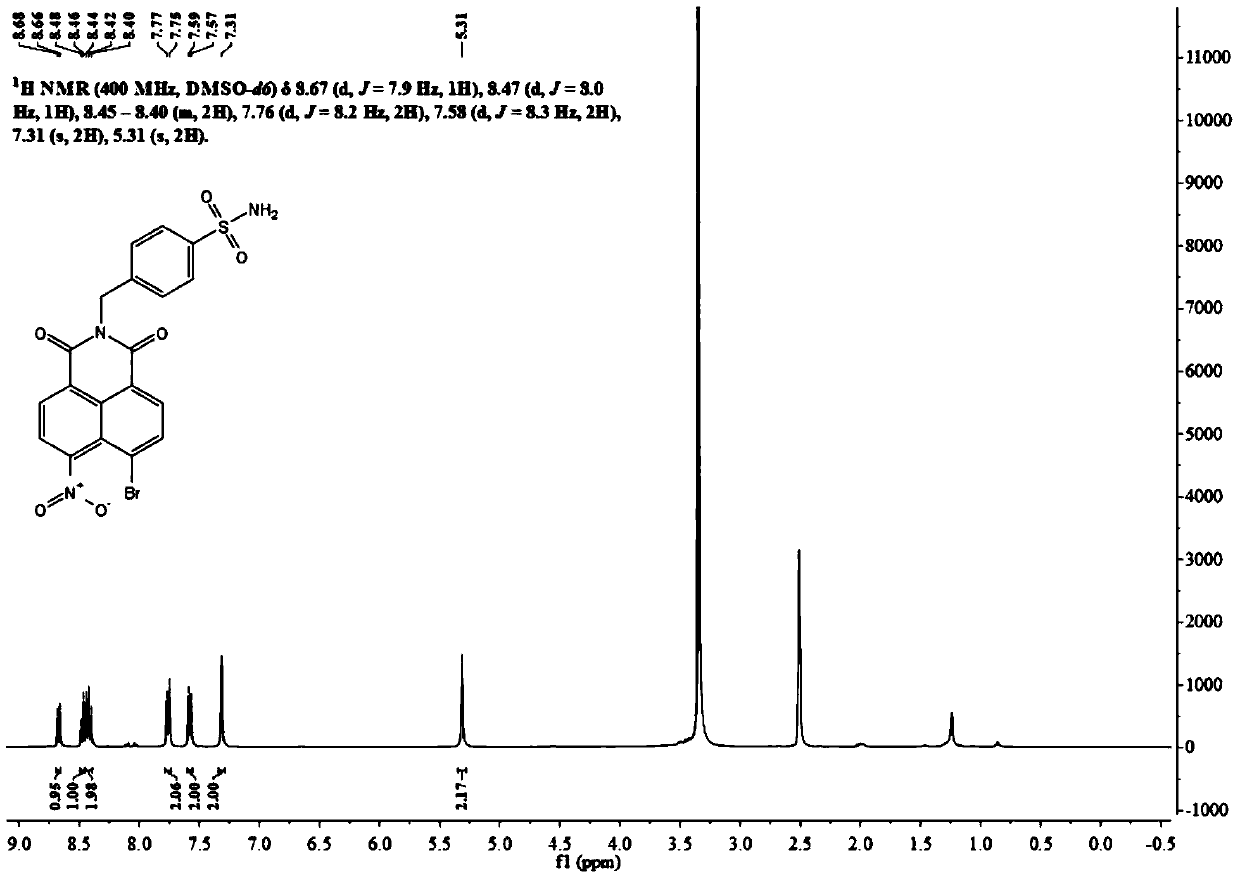
[0034] Its H NMR spectrum is as follows figure 1 As shown, the specific data are as follows:
[0035] 1 H NMR (400MHz, DMSO-d 6 )δ8.50(tdd,J=13.0,7.9,5.1Hz,2H),8.32–8.24(m,1H),8.20–8.10(m,1H),7.96(dd,J=8.4,7.4Hz,1H) ,7.77(d,J=8.1Hz,2H),7.56(d,J=8.3Hz,2H),7.33(s,2H),5.29(s,2H).
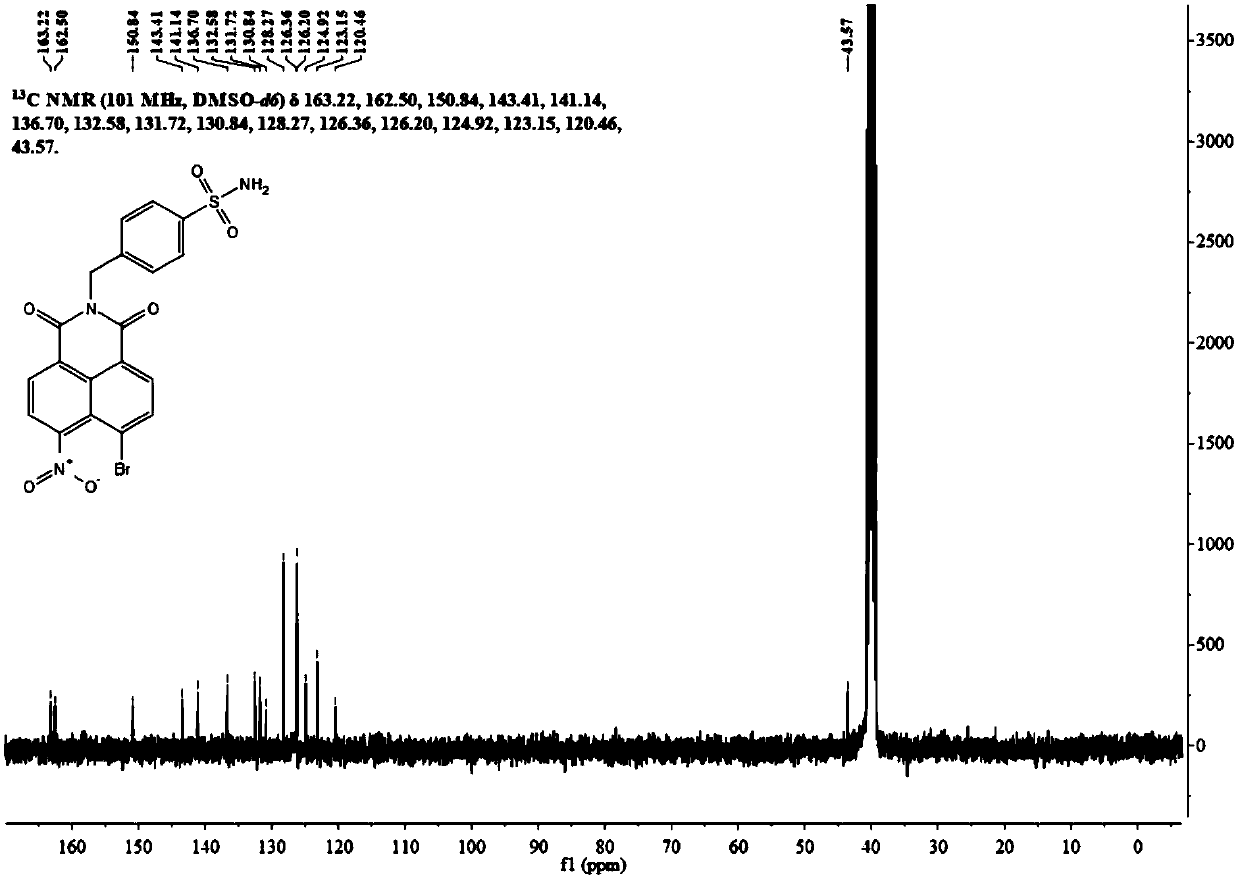
[0036] Its carbon NMR spectrum is as follows figure 2 As shown, the specific data are as follows:
[0037] 13 C NMR (101MHz, DMSO-d 6 )δ163.45, 163.4...
Embodiment 2
[0057] Synthesis of carbonic anhydrase probe SML-Aze.
[0058] The synthetic route and product structure of intermediate SML-Br are as follows:
[0059]
[0060] Add 4-bromo-1,8-naphthalene anhydride (1.0 g, 3.6 mmol), 4-(aminomethyl)benzenesulfonamide hydrochloride (0.9 g, 3.6 mmol) and triethylamine (1.0 g, 10.2mmol), and the reaction solution was heated to 60°C. After 10 h, the reaction solution was cooled to room temperature and the turbid solution was suction-filtered to obtain 0.8 g of off-white solid SML-Br, with a yield of 85%.
[0061] Its H NMR spectrum is as follows figure 1 As shown, the specific data are as follows:
[0062] 1 H NMR (400MHz, DMSO-d 6 )δ8.50(tdd,J=13.0,7.9,5.1Hz,2H),8.32–8.24(m,1H),8.20–8.10(m,1H),7.96(dd,J=8.4,7.4Hz,1H) ,7.77(d,J=8.1Hz,2H),7.56(d,J=8.3Hz,2H),7.33(s,2H),5.29(s,2H).
[0063] Its carbon NMR spectrum is as follows figure 2 As shown, the specific data are as follows:
[0064] 13 C NMR (101MHz, DMSO-d 6 )δ163.45, 163.41, ...
Embodiment 3
[0079] Synthesis of carbonic anhydrase probe SML-Aze.
[0080] The synthetic route and product structure of intermediate SML-Br are as follows:
[0081]
[0082] Add 4-bromo-1,8-naphthalene anhydride (3.0 g, 10.8 mmol), 4-(aminomethyl)benzenesulfonamide hydrochloride (7.2 g, 32.4 mmol) and triethylamine (13.6 g, 72mmol), and the reaction solution was heated to 80°C. After 6h, the reaction solution was cooled to room temperature and the turbid solution was suction-filtered to obtain 3.97 g of off-white solid SML-Br, with a yield of 81%.
[0083] Its H NMR spectrum is as follows figure 1 As shown, the specific data are as follows:
[0084] 1 H NMR (400MHz, DMSO-d 6 )δ8.50(tdd,J=13.0,7.9,5.1Hz,2H),8.32–8.24(m,1H),8.20–8.10(m,1H),7.96(dd,J=8.4,7.4Hz,1H) ,7.77(d,J=8.1Hz,2H),7.56(d,J=8.3Hz,2H),7.33(s,2H),5.29(s,2H).
[0085] Its carbon NMR spectrum is as follows figure 2 As shown, the specific data are as follows:
[0086] 13 C NMR (101MHz, DMSO-d 6)δ163.45, 163.41, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com