Preparation method of electrode material, electrode material, electrode and lithium ion battery
A technology of electrode materials and composite materials, applied in the field of batteries, can solve the problems of low electrode conductivity, etc., and achieve the effects of high ionic conductivity, easy mass production, and enhanced ionic conductivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
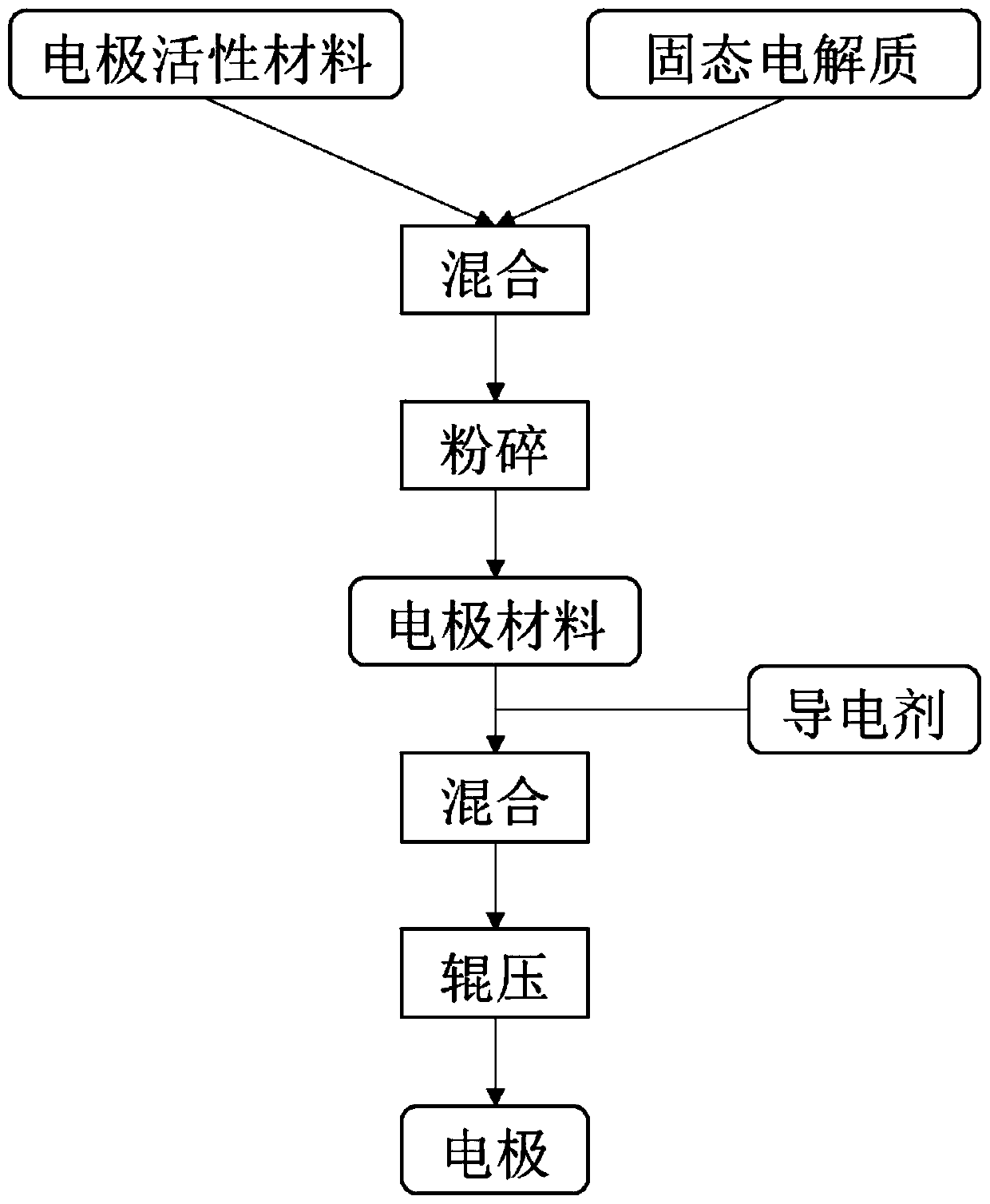
[0038] The invention provides a preparation method of an electrode material. It includes the following steps.
[0039] Step S1, mixing the electrode active material with the solid electrolyte or the precursor of the solid electrolyte and then granulating to obtain a composite material, wherein the solid electrolyte includes an organic solid electrolyte or an organic-inorganic composite solid electrolyte, and the organic-inorganic composite Solid electrolytes include organic solid electrolytes and inorganic solid electrolytes.
[0040] In this step, the purpose of firstly mixing the electrode active material with the solid electrolyte or the precursor of the solid electrolyte is to refine and fully contact the electrode active material with the solid electrolyte or the precursor of the solid electrolyte in advance. The electrolyte has a high ionic conductivity, so that it can fully contact the electrode active material first, which can effectively improve the ion transport per...
Embodiment 1
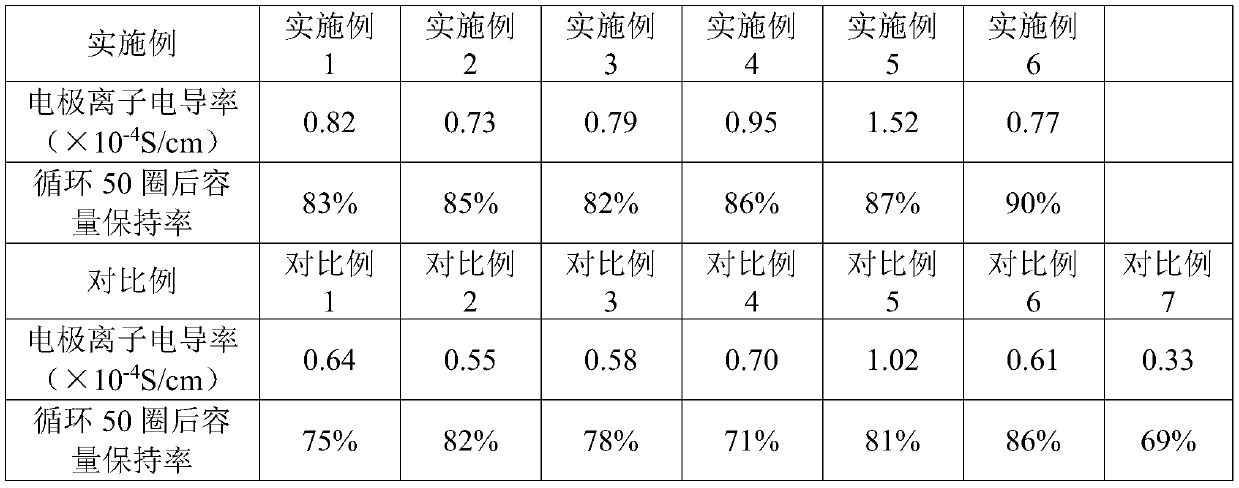
[0072] Embodiment 1: Lithium cobaltate positive electrode
[0073] (1) The positive electrode active material is lithium cobaltate positive electrode material with a median particle size of 5um; the lithium salt in the organic solid electrolyte selected is LiFSI, the polymer is PVDF-HFP, the plasticizer is EC, and the inorganic filler is Al 2 o 3 , the mass ratio of the lithium salt to the polymer is 5:1, the mass ratio of the plasticizer to the polymer is 60:100, and the mass proportion of the inorganic filler is 1%.
[0074] (2) Mixing by dry mixing method, the above-mentioned lithium cobaltate and organic solid electrolyte are mixed by ball milling at a weight ratio of 100:5, and after mixing, they are mechanically pulverized to form an active material-electrolyte composite material.
[0075] (3) The above-mentioned active material-electrolyte composite material and conductive carbon black Super-P were uniformly mixed according to the weight ratio of 100:10, and the electr...
Embodiment 2
[0076] Embodiment 2: graphite negative electrode
[0077] (1) The negative electrode active material is a graphite negative electrode material with a median particle size of 8um; in the selected organic-inorganic composite solid electrolyte, the lithium salt in the organic electrolyte is LiTFSI, the polymer is PEO, the plasticizer is DMC, and no The mass ratio of inorganic filler, lithium salt and polymer is 1:20, the mass ratio of plasticizer to polymer is 5:100; the inorganic electrolyte is lithium titanium aluminum phosphate. The mass ratio of organic electrolyte and inorganic electrolyte is 10:1.
[0078] (2) Mixing by wet mixing method, the above-mentioned graphite and organic-inorganic solid electrolyte are mixed in a kneading machine according to a weight ratio of 100:10, and tetrahydrofuran is selected as a solvent. After mixing, the solvent is heated to volatilize, and then mechanically pulverized to form an active material-electrolyte composite material.
[0079] (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Median particle size | aaaaa | aaaaa |
| Median particle size | aaaaa | aaaaa |
| Median particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com