Compound for reducing lipotoxic damage
A technology of compounds and compositions, applied in the field of compounds for reducing lipotoxic damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] Example 1. Preparation and Testing of Unformylated Amino Acid Analogs
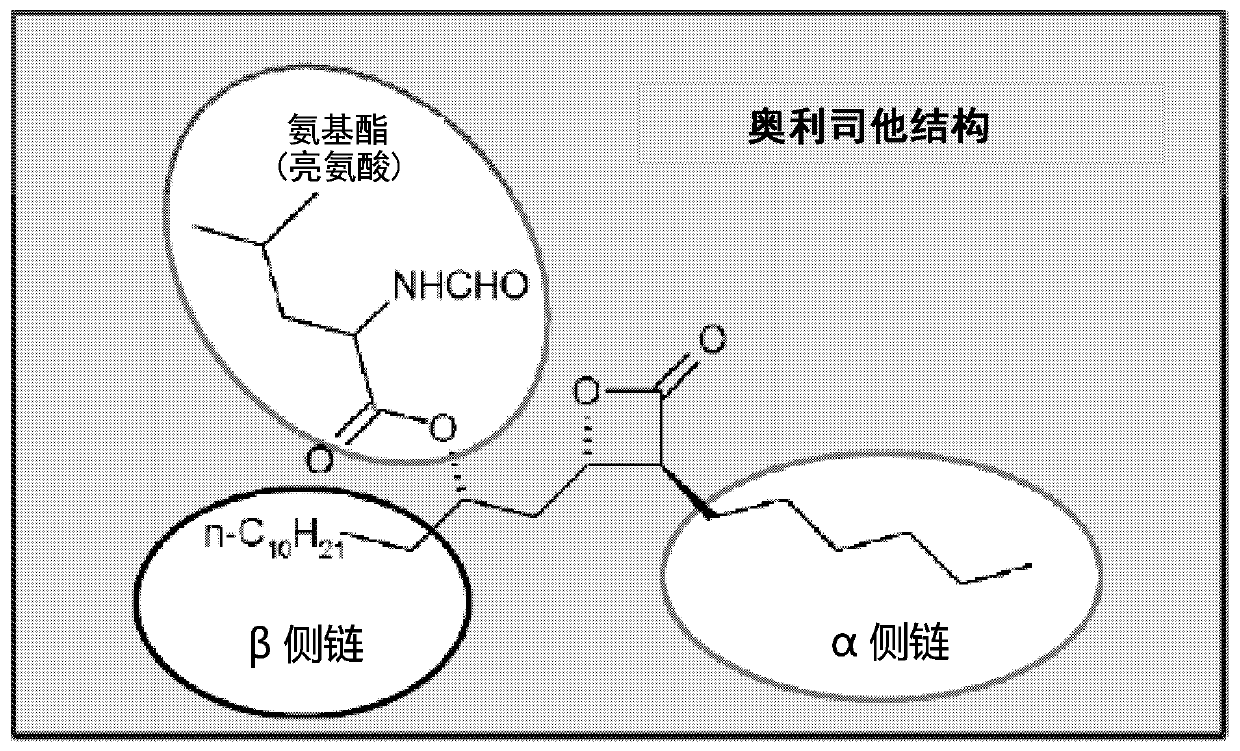
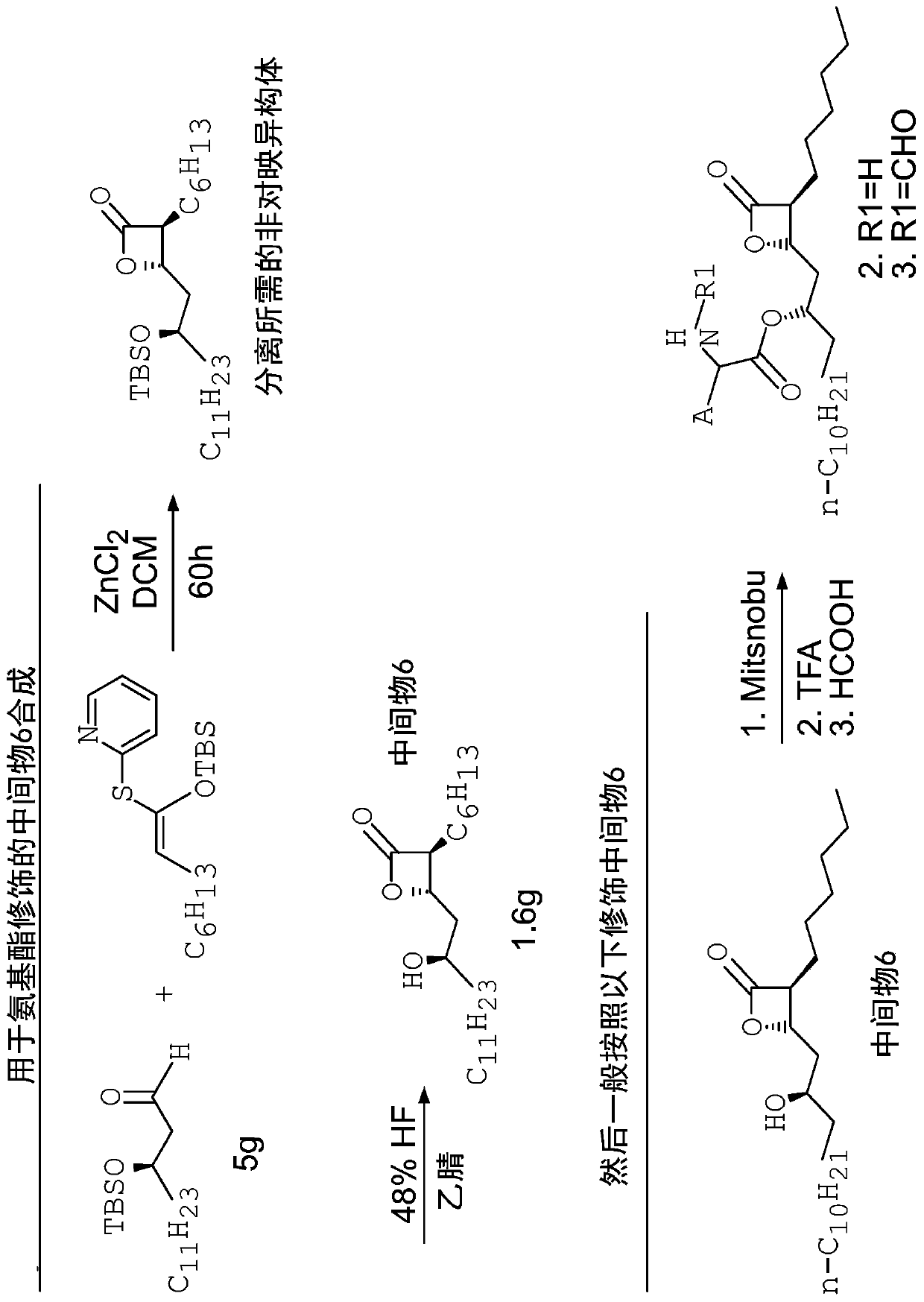
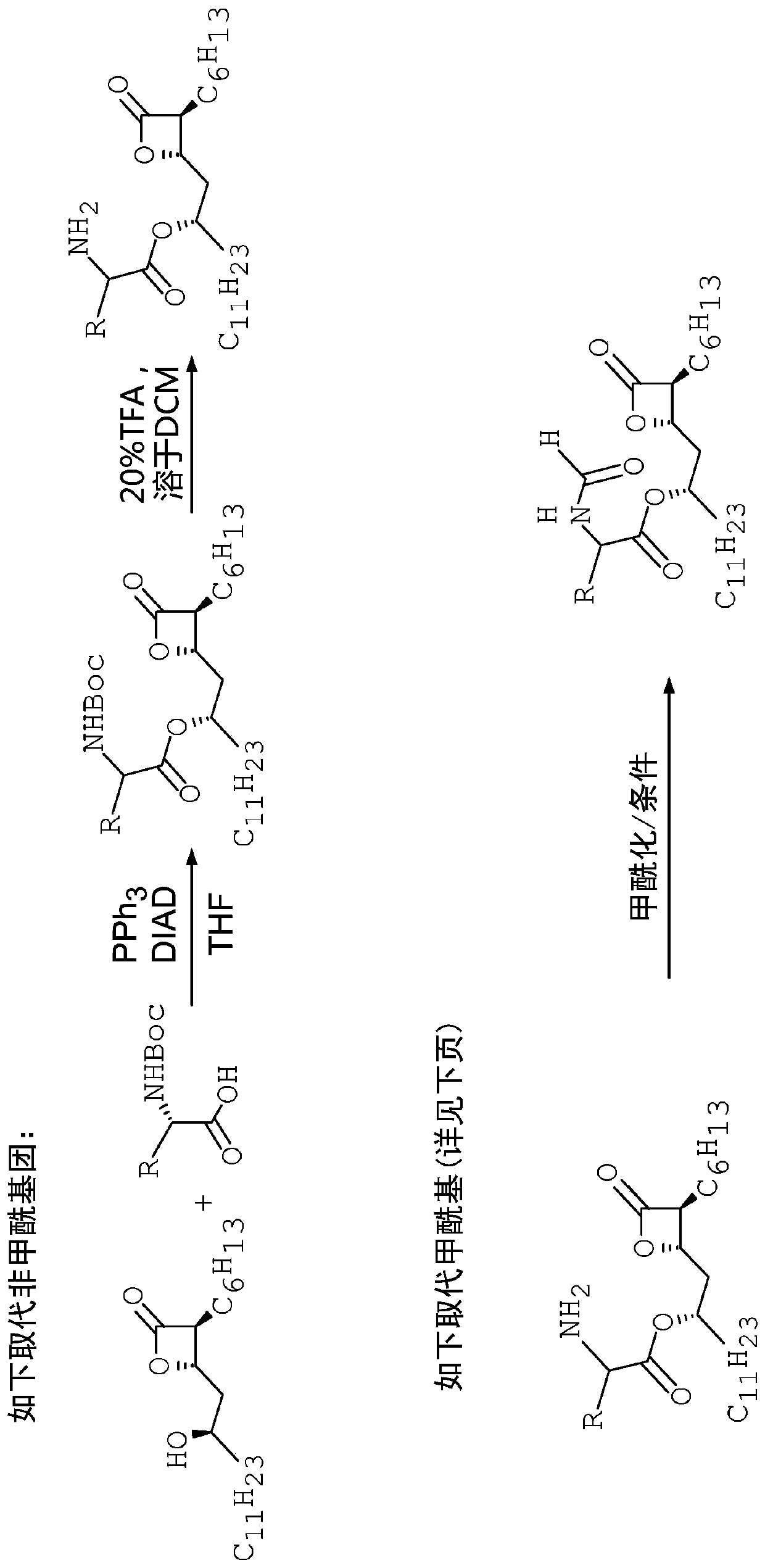
[0114] A number of unformylated amino acid analogs were prepared in which the L-amino ester leucine was replaced by a congener. The synthetic steps of this method are as follows figure 2 shown. Such as image 3 As shown, the resulting compounds include
[0115] Compounds wherein the amino ester of L-leucine (#723, is orlistat) is substituted by a congener such as N-methylleucine (#724) or a more polar or shorter congener, including L -Alanine (#716), N-Acetyl-L-Cysteine (#717), L-Asparagine (#718), L-Histidine (#725), L-Methionine Acid Sulfone (#726), L-Arginine (727), L-Diaminopimelic Acid (#728), L-Serine (#729), L-Ornithine (#731), or N-Acetyl base leucine (#732).
[0116] To test the potency of these compounds, drugs were dissolved in ethanol as 20 mM stock solutions and then diluted into PBS as 600 micromolar working stocks (3.3% ethanol). This is used right away or left at room tem...
Embodiment 2
[0117] Example 2. Preparation and testing of formylated amino acid analogs
[0118] A number of formylated amino acid analogs were prepared in which the L-amino ester leucine was replaced by a congener. The synthetic steps of this method are as follows figure 2 and Figure 5 shown.
[0119] These drugs (#733,734,738,739,740,742,743, as Figure 6 indicated) was initially tested as described in Example 1. An approximately 10-fold increase in stability and potency after overnight storage was noted for compounds 741, 736 and 743 compared to orlistat (Figure 7). Compounds were further tested for their efficacy in reducing lipotoxic injury to acinar cells in acinar-adipocyte co-cultures as described in the following: Navina S, Acharya C, DeLany JP, Orlichenko LS, Baty CJ, Shiva SS, Durgampudi C, Karlsson JM, Lee K, Bae KT et al.: Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity ( Lipotoxicity causes multisystem organ failure and e...
Embodiment 3
[0120] Example 3. Preparation and testing of beta chain analogs.
[0121] To further test options for improving the potency of the analogs, the β-strand of 741 was altered to make it more hydrophilic. For this, if Figure 9-14 Compounds 760, 762, 763 were prepared as described. Modification at this position did not improve lipase inhibition and in fact a decrease in potency was observed. see Figure 15 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com