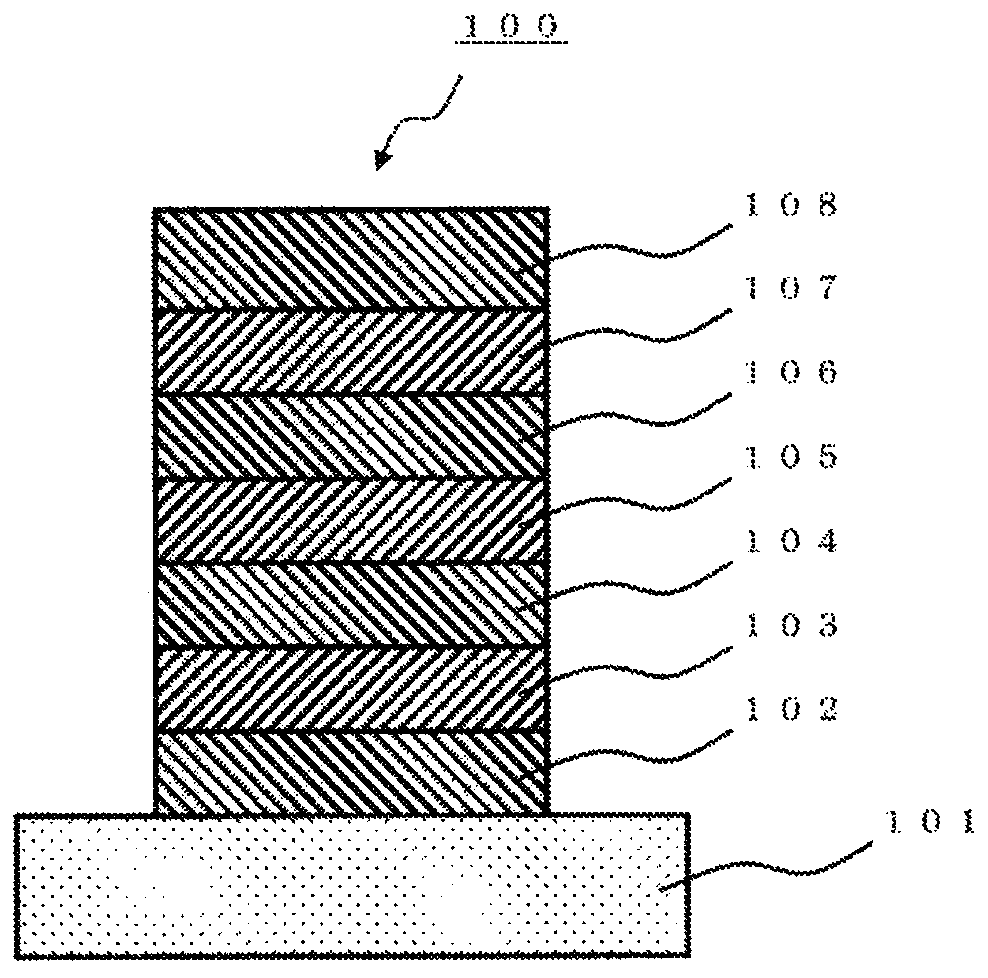
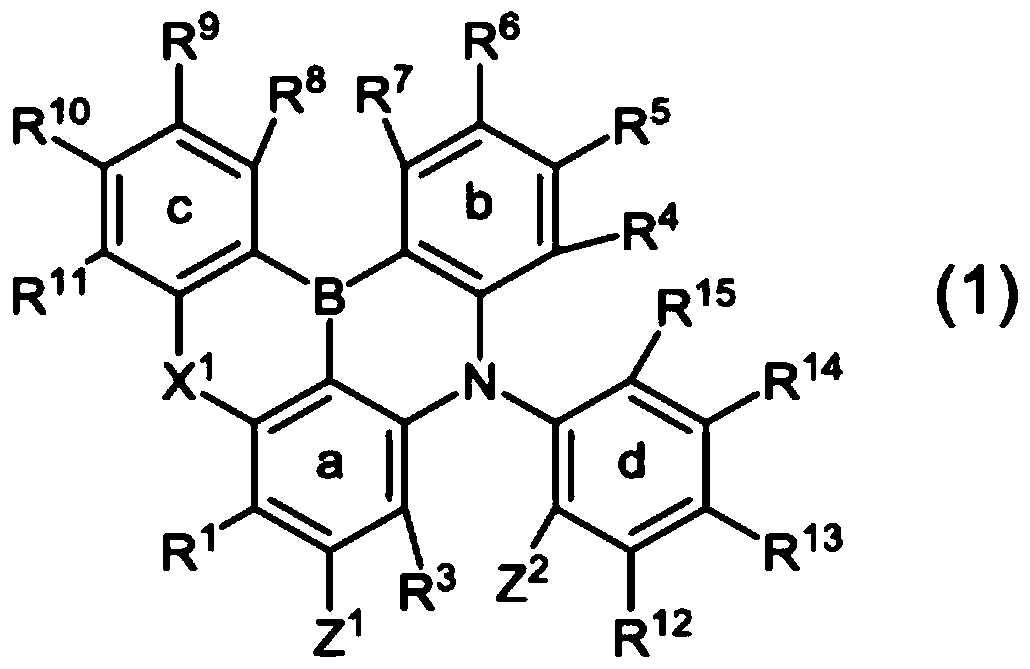
Material for organic device and organic electroluminescent element using same
A technology of organic components and organic electric fields, which is applied in the field of organic field-effect transistors and organic thin-film solar cells, and can solve problems such as unknown characteristics and different electronic states
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0567] Hereinafter, although an Example demonstrates this invention more concretely, this invention is not limited to these.
[0568] First, a synthesis example of the polycyclic aromatic compound of the present invention will be described below.
[0569] [chem 95]
[0570]
[0571] [chem 96]
[0572]
[0573] [chem 97]
[0574]
[0575] [chem 98]
[0576]
Synthetic example (1
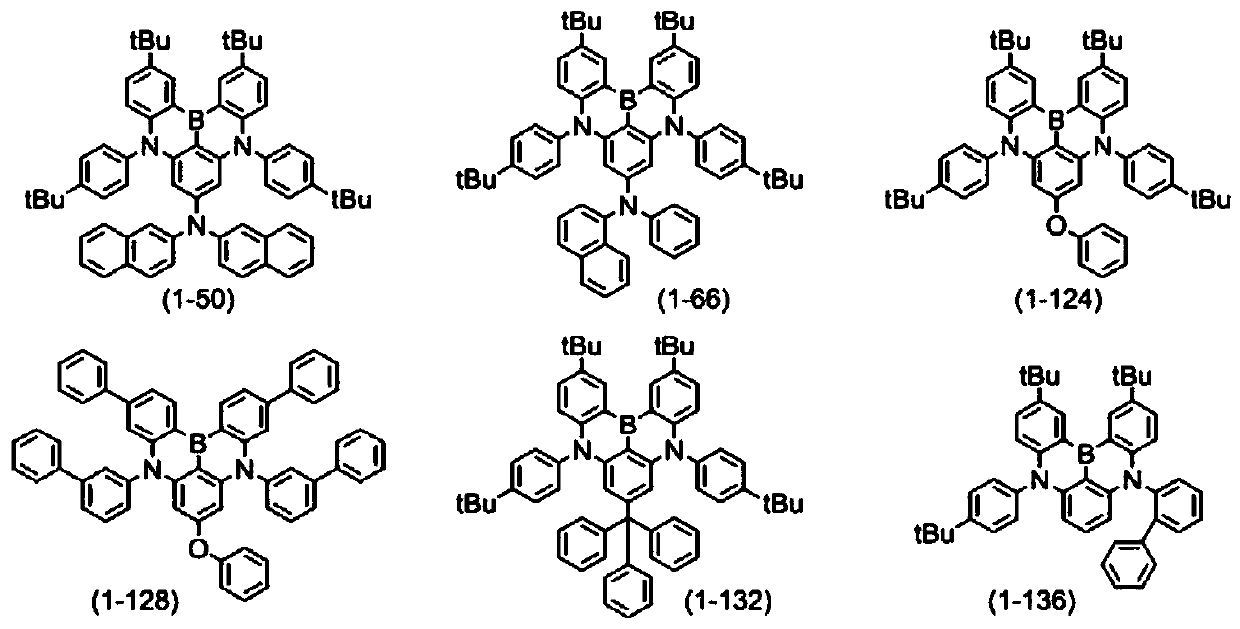
[0578] Synthesis of compound (1-50)
[0579] [chem 99]
[0580]
[0581] Under nitrogen atmosphere, put 3,4,5-trichloroaniline (7.0g), 2-bromonaphthalene (22.0g), dichlorobis[(di-tert-butyl(4- A flask of dimethylaminophenyl) phosphino)] palladium (Pd-132, 0.25g), sodium tert-butoxide (NaOtBu, 8.6g) and xylene (130ml) was heated and stirred at 130°C for 1 hour, After cooling the reaction liquid to room temperature, water and ethyl acetate were added and liquid-separated. After washing the organic layer with water, the solvent was distilled off under reduced pressure. Thereafter, the intermediate (A) (15.0 g) was obtained by refining with a silica gel column (eluent: toluene / heptane=1 / 9 (volume ratio)).
[0582] [chemical 100]
[0583]
[0584] Under a nitrogen atmosphere, put intermediate (A) (15.0g), two (4-(tert-butyl) phenylamine) (20.7g), two (dibenzylidene acetone) palladium (0) (Pd(dba) 2 , 0.38g), 2-dicyclohexylphosphino-2', 6'-dimethoxybiphenyl (SPhos, 0.69g...
Synthetic example (2
[0593] Synthesis of compound (1-66)
[0594] [chem 103]
[0595]
[0596] Under nitrogen atmosphere, put 3,4,5-trichloro-N-phenylaniline (10.0g), 1-bromonaphthalene (9.1g), Pd-132 (0.26g), NaOtBu (5.3g ) and xylene (75ml), heated and stirred at 100°C for 1 hour. After cooling the reaction liquid to room temperature, water and ethyl acetate were added and liquid-separated. After washing the organic layer with water, the solvent was distilled off under reduced pressure. Thereafter, purification was performed using a silica gel short path column (eluent: toluene). Further, reprecipitation was carried out with heptane to obtain an intermediate product (C) (11.0 g).
[0597] [chemical 104]
[0598]
[0599] Under a nitrogen atmosphere, put intermediate (C) (11.0g), two (4-(tert-butyl) phenyl) amine (17.1g), Pd (dba) 2 (0.32g), SPhos (0.57g), NaOtBu (6.6g) and xylene (90ml) were heated and stirred at 110°C for 1 hour. After cooling the reaction liquid to room temperatur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electron work function | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com