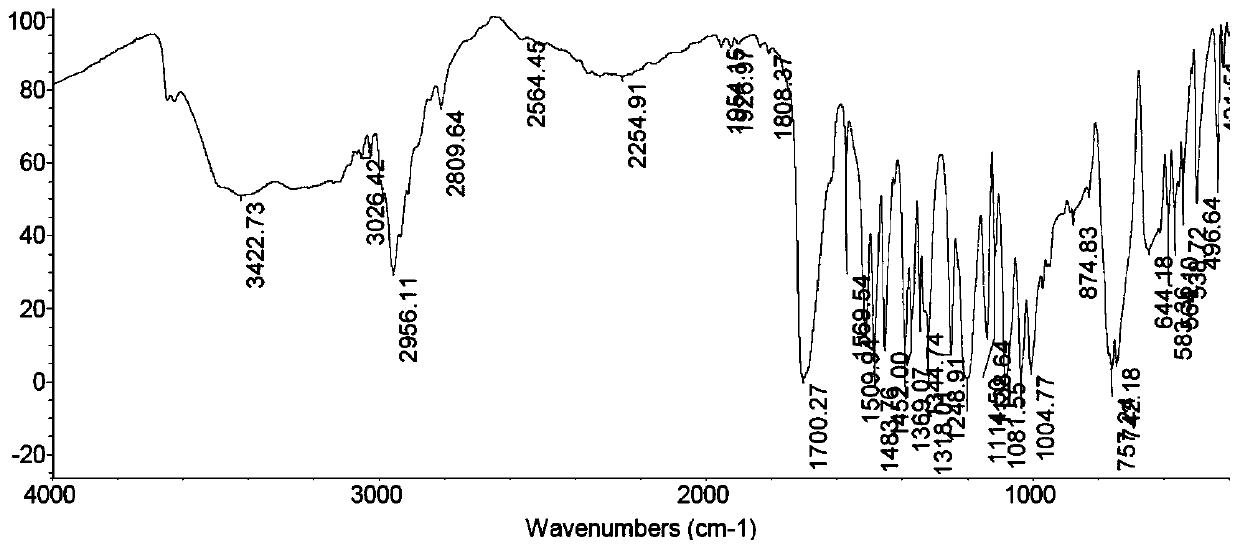
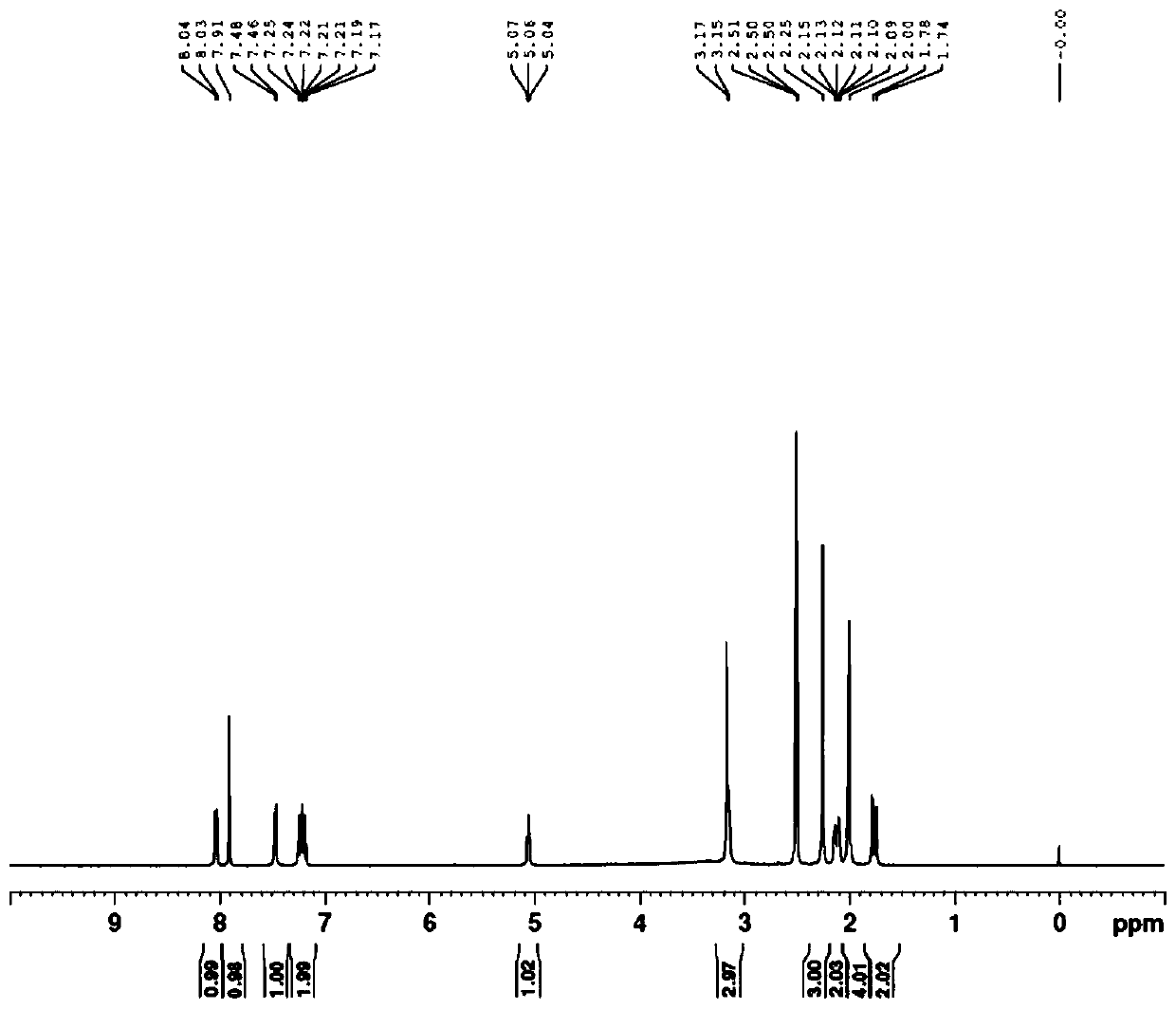
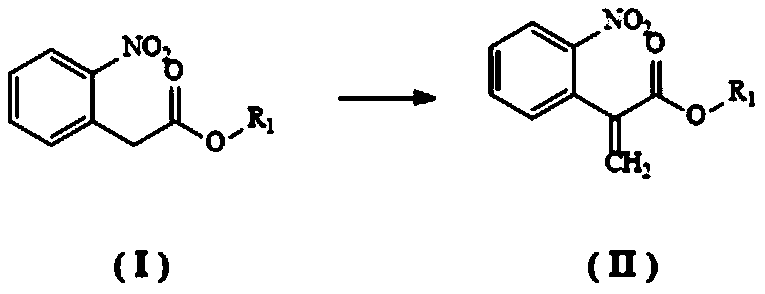
Synthesis method of N-hydroxy tropisetron
A technology of hydroxytropane and a synthetic method, applied in the field of drug synthesis, can solve problems such as unreported, and achieve the effects of strong operability, reasonable process design and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The synthetic method of N-hydroxytropisetron comprises the following steps:
[0055] (1) Synthesis of Intermediate II
[0056] The reaction process is:
[0057]
[0058] Get 20 grams of benzyl 2-nitrophenylacetate and dissolve it in 400 milliliters of dioxane, add 5.01 grams of tetrabutylammonium bisulfate, 20.38 grams of potassium carbonate and 6.64 grams of paraformaldehyde, then heat the mixture to 100°C for reaction 4 hours. After the reaction solution was cooled to room temperature, it was filtered, and the filtrate was partially concentrated and purified by column chromatography to obtain 15.65 g of an oily substance, namely Intermediate II, with a yield of 74.9%.
[0059] (2) Synthesis of Intermediate III
[0060]
[0061] The reaction process is:
[0062] Dissolve 15 grams of intermediate II in 600 milliliters of tetrahydrofuran, add 40.16 grams of anhydrous stannous chloride and 77.86 grams of anhydrous potassium acetate, use mechanical stirring, reac...
Embodiment 2
[0080] The synthetic method of N-hydroxytropisetron comprises the following steps:
[0081] (1) Synthesis of Intermediate IIa
[0082] The reaction process is:
[0083]
[0084] Get 20 grams of tert-butyl 2-nitrophenylacetate and dissolve in 120 milliliters of toluene, add 1.56 grams of tetrabutylammonium iodide, 8.94 grams of sodium carbonate and 9 milliliters of 37% aqueous formaldehyde solution, then the mixture is heated to 115 ° C to react 8 Hour. After the reaction solution was cooled to room temperature, it was filtered, and the filtrate was extracted with ethyl acetate. The organic phase was dried and concentrated over anhydrous sodium sulfate, and purified by column chromatography to obtain 6.40 g of an oily substance, namely intermediate IIa, with a yield of 60.9%.
[0085] (2) Synthesis of Intermediate IIIa
[0086]
[0087] The reaction process is:
[0088] Dissolve 6.4 grams of intermediate IIa in 128 milliliters of ethyl acetate, add 3.6 grams of reduce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com