Soluble recombinant tartary buckwheat metallothionein FtMT with cell-penetrating activity and preparation method thereof
A metallothionein, soluble technology, applied in the field of soluble recombinant buckwheat metallothionein FtMT and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Plasmid vector and transmembrane peptide gene modification: Escherichia coli alkaline phosphatase (phoA) gene promoter is inhibited in the presence of excess phosphate, and protein expression is gradually suppressed under starvation (bacterial growth enters the lag phase) Induction, through the phoA promoter controlled by the phosphate concentration to achieve the purpose of gradual chronic expression, reducing the chance of overloading the bacterial expression system to form inclusion bodies, and slow accumulation also alleviates the toxicity of exogenous proteins that are produced during rapid accumulation , gain expression.
[0028] The signal peptide modified pel B is modified from the signal peptide of pectinase pel B, which has higher secretion efficiency and can guide foreign proteins to enter the periplasm through the membrane, significantly reducing the cytotoxicity and inhibition that affect the normal physiological functions of the host cells. The ...
Embodiment 2
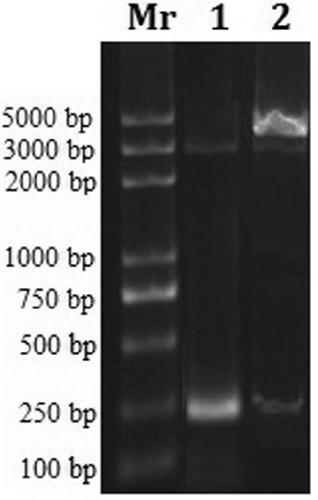
[0033] Example 2: Construction and identification of recombinant expression plasmids: TatM-FtMT was synthesized by chemical synthesis in vitro according to the tartary buckwheat metallothionein FtMT coding region gene and TatM coding sequence. Using TatM-FtMT as template, specific primer: phoA-TatM-FtMT-F: 5′-G CCATGG CCTATGGCAGGAAGAAG-3′ (with NCOI cleavage site); phoA-TatM-FtMT-R: 5′-A GGATCC GCAGTTGCAGGGATTGC-3' (containing BamH I restriction site), PCR amplification.
[0034] The PCR reaction conditions were: pre-denaturation at 94°C for 5 min; denaturation at 94°C for 30 s; annealing at 65°C for 30 s; extension at 72°C for 1 min, 30 cycles. Theoretical size of TatM-FtMT amplified products was 280 bp. The PCR product was recovered after detection and identification by 15g / L agarose gel electrophoresis.
[0035] The PCR product and phoA expression vector were double-digested with Nco I and BamH I, and the digested PCR fragment and phoA plasmid were respectively r...
Embodiment 3
[0037] Example 3: Expression and purification of TatM-FtMT induced by recombinant protein:
[0038] The correct recombinant expression vector identified by sequencing was transformed into competent peptide Escherichia coli BL21 (DE3), and the recombinant engineering strain phoA-TatM-FtMT / BL21 (DE3) was constructed. The engineered bacteria were inoculated in LB medium, shaken at 37°C and 200 r / min overnight for 12 h to prepare seeds. The next day, according to the inoculation amount of 2.5% (v / v), inoculate them into the optimized and improved Neidhardt low phosphorus medium respectively, and the medium components are as follows: 0.25g / L enzyme mother extract, 2.5g / L tryptone, 3g / L L (NH4) 2 SO 4 , 5g / L NaCl, 1g / L MgSO 4 , 4g / L glucose, phosphorus content 0.05mmol / L. Add 500 µmol / LZnSO after 30 min of low phosphorus culture 4 Stabilize protein to prevent self-polymerization, induce culture at 30°C, 180r / min.
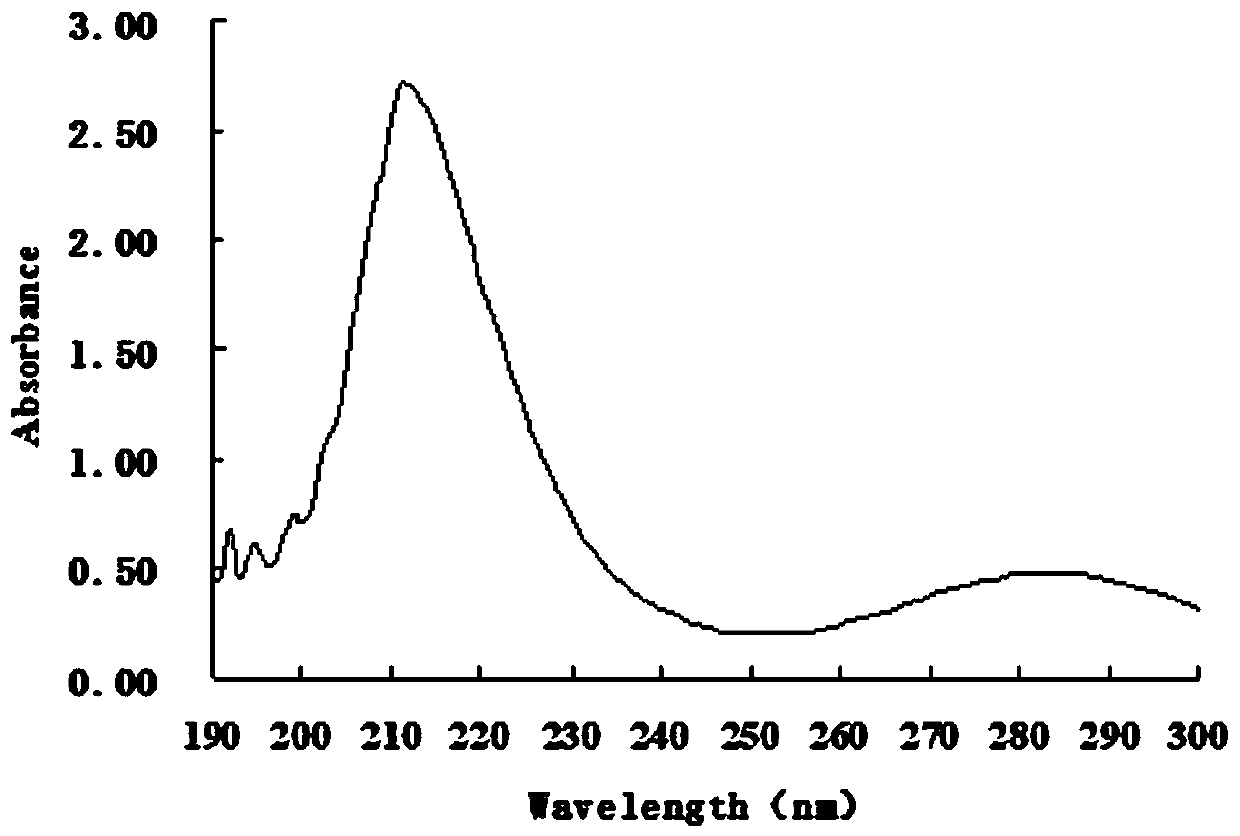
[0039] Phosphorus content was measured using a phosphorus dete...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com