Recombinant human insulin and purification and preparation method thereof
A recombinant human insulin, ubiquitin-like technology, applied in the field of biomedicine, can solve the problems of high production cost, low renaturation rate of inclusion body expression, complicated production process, etc., to reduce production cost, realize soluble expression, and solve complex problems. low sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Construction of recombinant human proinsulin gene expression vector
[0027] The recombinant human proinsulin gene sequence having the gene sequences of SEQ ID NO.1, SEQ ID NO.2, SEQ ID NO.3, and SEQ ID NO.4 was synthesized by a DNA synthesis company (Invitrogen).
[0028] Ligate the synthetic recombinant human proinsulin target fragment with the prokaryotic expression vector pET30a(+) (the vector has been connected with the SUMO gene), and the ligation reaction system (10 μl) is as follows:
[0029]
[0030] A total of 10 μl of the system was mixed, and connected overnight at 4°C. After enzyme digestion and identification, the recombinant human proinsulin gene expression vector pET-30a-SUMO-Insulin was constructed.
Embodiment 2
[0032] Induced Expression of Recombinant Human Proinsulin Fusion Protein
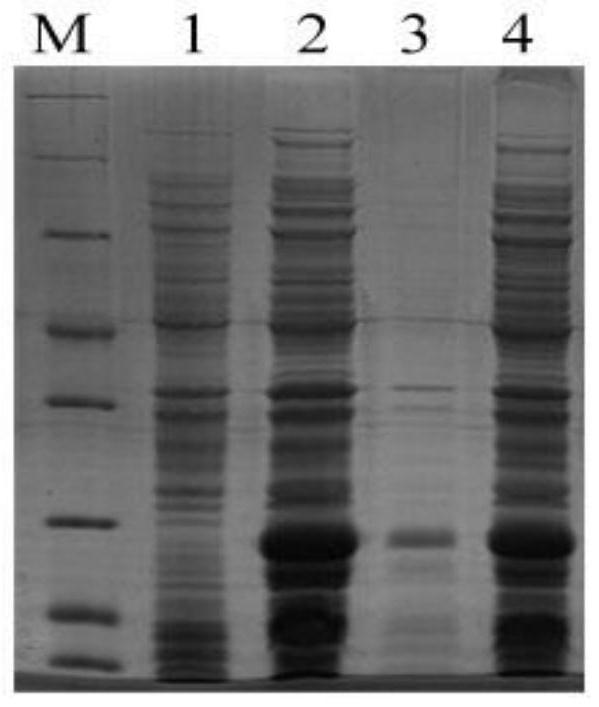
[0033] The constructed recombinant human proinsulin gene expression vector pET-30a-SUMO-Insulin was transformed into the expression strain Rosetta (DE3) (Beijing Quanshijin Biotechnology Co., Ltd., catalog number: CD801). Transformed single colonies were inoculated into 20 mL of LB medium containing ampicillin (Amp) (100ug / mL), cultured at 37°C for 8 hours, and then inoculated into another 20 mL of LB containing Amp (100ug / mL) at a ratio of 1:100. culture medium at 37°C, when the A600 is around 0.35, add isopropylthiogalactopyranoside (IPTG) to a final concentration of 0.25mmol / L for induction, the induction temperature is 20°C, harvest the cells after 4h, and use lysisbuffer (20mM Na 3 PO 4 , 20mM imidazole, 500mM NaCl, pH 7.5) to resuspend the bacterium, centrifuge after breaking the bacterium, take the supernatant and precipitate respectively for 12% SDS-PAGE electrophoresis analysis. The results ...
Embodiment 3
[0035] Purification and separation of insulin analogues
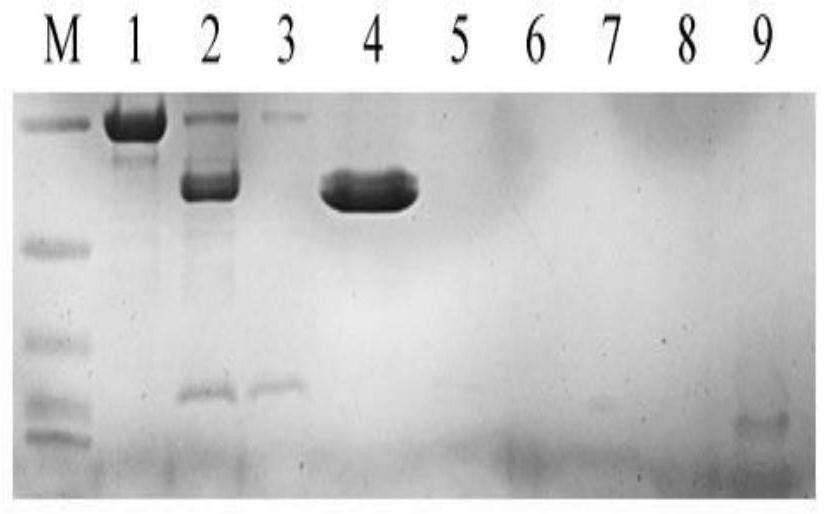
[0036] According to the conditions for the induced expression of the recombinant human proinsulin fusion protein in Example 2, expand the culture, collect the bacteria after induction and expression, suspend with lysis buffer, sonicate and then centrifuge or filter to obtain the supernatant of the bacteria.
[0037] The above supernatant was purified by affinity chromatography, Ni Sepharose 6FF was used as the chromatography medium, and the equilibrium liquid was lysis buffer. The eluent is 20mM Na 3 PO 4 , 250mM imidazole, 500mM NaCl, pH 7.5, to obtain a crude sample of SUMO-Insulin fusion protein.
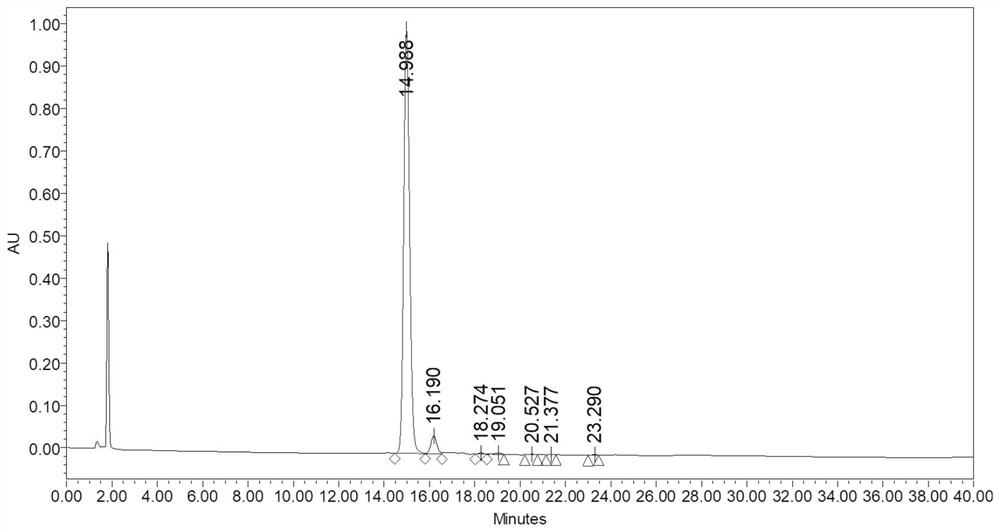
[0038] Add trypsin to the crude sample of SUMO-Insulin fusion protein obtained above and mix for enzymatic digestion reaction, so that the mass ratio of trypsin and crude SUMO-Insulin fusion protein is 1:1000, react the mixture at 37°C for 1 h, and use Phosphoric acid was used to adjust the pH of the mixture to approxim...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com