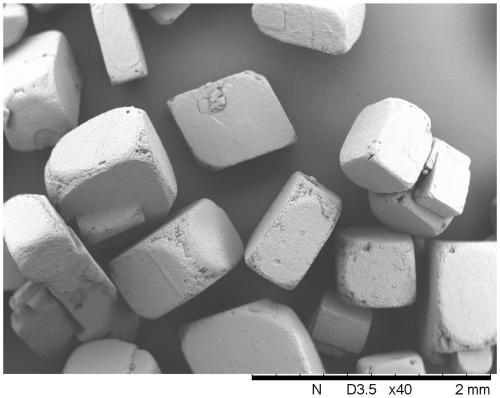
Method for preparing cubic anhydrous betaine crystals
A technology of anhydrous betaine and cubic shape, which is applied in the field of preparation of cubic anhydrous betaine crystals, and can solve problems such as the concentration of anhydrous betaine crystals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0049] Add 91.9g of anhydrous betaine powder and 118g of anhydrous methanol to the crystallizer, and the ratio of anhydrous betaine powder to solvent is 0.78:1. Set the stirring speed to 400r / min, and raise the temperature at a heating rate of 7°C / min to dissolve the powder, and the temperature of the solution rises to 72.3°C, reaching the boiling point of the crystallization system.
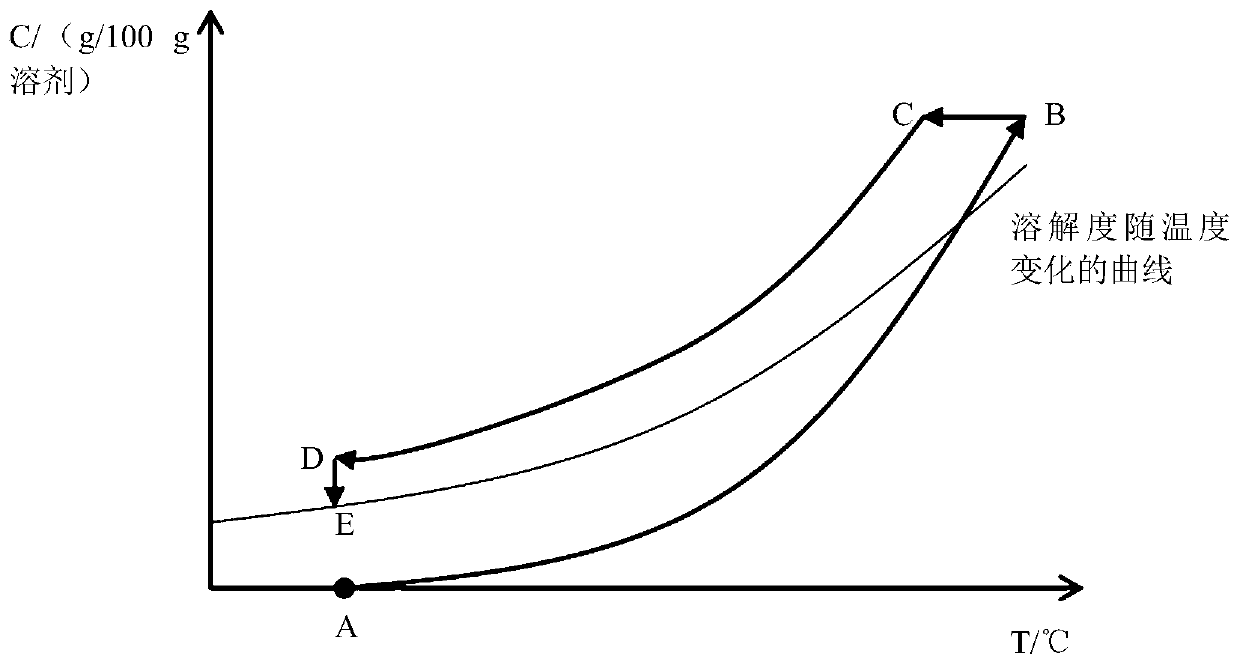
[0050] Cool down rapidly, the cooling rate is 0.3°C / min, when the solution temperature drops to 71.9°C, crystal nuclei begin to precipitate, at this time no anhydrous betaine powder is added, that is, the dosage is 0g / g solvent, and the solution explodes crystal nucleus, and then continue to cool down to room temperature at a cooling rate of 0.05°C / min.
[0051] After the temperature of the solution drops to 20° C., first filter, then wash the crystals with an acetone-methanol solution with an acetone mass fraction of 60 wt %, and then wash the crystals with acetone, the washing process needs to b...
Embodiment example 2
[0053] Add 91.9g of anhydrous betaine powder and 118g of methanol into the crystallizer, and the mass ratio of anhydrous betaine powder to solvent is 0.78:1. Set the stirring speed to 400r / min, and raise the temperature at a heating rate of 6°C / min to dissolve the powder, and the temperature of the solution rises to 72.4°C, reaching the boiling point of the crystallization system.
[0054] Cool down rapidly at a cooling rate of 0.5°C / min. When the solution temperature drops to 72°C, crystal nuclei begin to precipitate. At this time, 1g of anhydrous betaine powder is added to the solution, that is, the dosage is 0.008g / g solvent, and the solution is Immediately, crystal nuclei were precipitated explosively, and then continued to cool down to room temperature at a cooling rate of 0.05°C / min.
[0055] When the temperature of the solution drops to 20° C., filter first, then quickly wash the crystals with an acetone-methanol solution with an acetone mass fraction of 60 wt%, and the...
Embodiment example 3
[0057] Add 92.5g of anhydrous betaine powder and 118g of methanol into the crystallizer, and the mass ratio of anhydrous betaine powder to solvent is 0.78:1. Set the stirring speed at 400r / min, and raise the temperature at a rate of 5°C / min to dissolve the powder, and the temperature of the solution rises to 73.2°C, reaching the boiling point of the crystallization system.
[0058] Cool down rapidly at a rate of 0.5°C / min. When the temperature of the solution drops to 73°C, crystal nuclei begin to precipitate. At this time, 0.5g of anhydrous betaine powder is added to the solution, that is, the dosage is 0.004g / g solvent. Crystal nuclei were precipitated explosively in the solution immediately, and then continued to cool down to room temperature at a cooling rate of 0.05°C / min.
[0059] When the temperature is lowered to 20° C., filter first, then quickly wash the crystals with an acetone-methanol solution with an acetone mass fraction of 60 wt%, and then immediately wash the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com