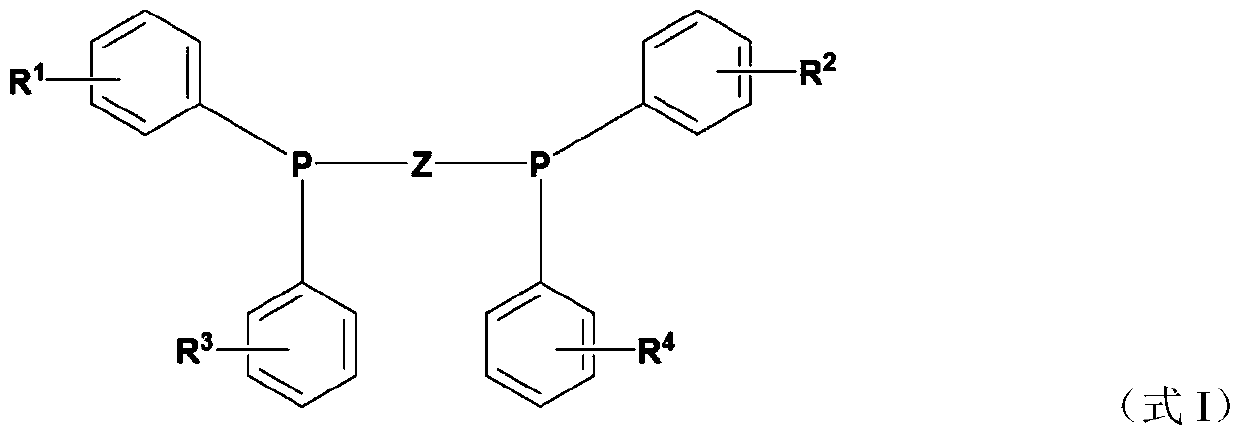
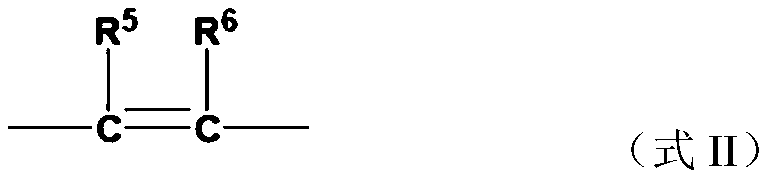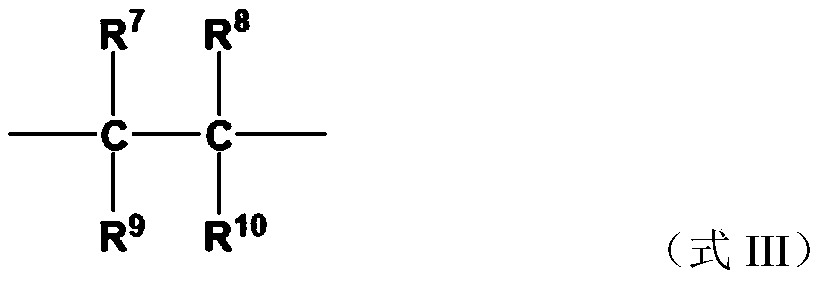Halogen-containing compound, application, catalyst composition, ethylene oligomerization method, ethylene trimerization method and ethylene tetramerization method
A compound, a halogen-containing technology, applied in the ligand of ethylene oligomerization catalyst composition, ethylene tetramerization field, can solve the problems such as unfavorable process economy, achieve high catalytic activity, good industrial application prospect and economic value, high stability sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0116] Preparation Example 1 is used to prepare halogen-containing compound I 1 .
[0117]
[0118] Halogen compound I 1 The preparation method refers to the above reaction formula, and the specific steps are as follows.
[0119] Under nitrogen protection, add n-butyllithium (11mmol) (6.6mL n-butyllithium hexane solution, the concentration of n-butyllithium is 1.6M) to 15mL of dry tetrahydrofuran in a reaction flask, cool to 0°C, stir 2.2 g (10 mmol) of difluorophenylphosphine chloride was added, and then acetylene (11 mmol) was added. After stirring for 0.5 h, the temperature was raised to room temperature (25° C., the same below), and stirring was continued for 2 h. Catalytic amounts of CuI and cesium carbonate were added, followed by 2.2 g (10 mmol) of difluorophenylphosphine chloride, the temperature was raised to 90° C. and stirred at 90° C. for 4 h. After the reaction, cool to room temperature, filter the reaction mixture, vacuum the filtrate to dryness, and pass t...
preparation example 2
[0123] Preparation example 2 is used to prepare halogen-containing compound I 2 .
[0124] In this preparation example, the halogen-containing compound was prepared by the same method as in preparation example 1, except that difluorophenylphosphine chloride was replaced by dichlorophenylphosphine chloride. The prepared compound is carried out nuclear magnetic resonance analysis, confirms that the prepared compound is the compound shown in formula I, wherein, R 1 , R 2 , R 3 and R 4 Both chlorine, R 5 and R 6 Both are hydrogen.
[0125] h 1 NMR (400MHz, CDCl 3 ): δ=7.30-7.00 (m, 16H), 5.18 (s, 2H).
preparation example 3
[0127] Preparation example 3 is used to prepare halogen-containing compound I 3 .
[0128]
[0129] Halogen compound I 3 The preparation method refers to the above reaction formula, and the specific steps are as follows.
[0130] Under nitrogen protection, tert-butylacetylene (11mmol) and 15mL of dry tetrahydrofuran were added to a 50mL reaction flask, and then n-butyllithium (11mmol) (6.6mL of n-butyllithium in hexane was added dropwise at 0°C, n The concentration of butyllithium is 1.6M). After the dropwise addition, continue stirring at 0°C for 30 min, then add 2.2 g (10 mmol) of difluorophenylphosphine chloride dropwise, after the dropwise addition, raise the temperature to room temperature (25°C, the same below), and continue stirring for 2 h. Catalytic amounts of CuI and cesium carbonate were added, followed by 2.2 g (10 mmol) of difluorophenylphosphine chloride, the temperature was raised to 90° C. and stirred at 90° C. for 4 h. After the reaction, cool to room t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com