Synthesis method of Teneligliptin dimer impurity
A technology of tiagliptin and synthetic method, applied in the field of synthesis of tiagliptin dimer impurities, achieving the effects of reasonable process design, easy availability of raw materials, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] A kind of synthetic method for Geglitin dimer impurity, comprises the following steps:
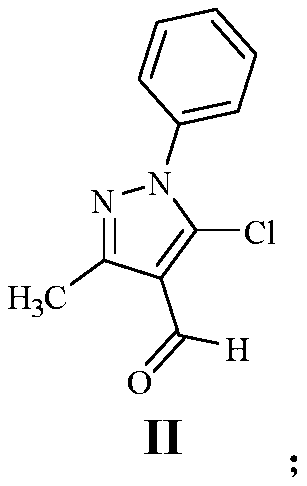
[0054] (1) Dissolve 10.00g of edaravone in tetrahydrofuran, add 12.32g of thionyl chloride dropwise at room temperature, stir at 80°C for 5 hours to obtain a brown solution, spot the plate to monitor the complete reaction; pour the reaction solution slowly Quenched in ice water, a solid product precipitated out of the water, the water phase was removed by filtration, and the filter cake was vacuum-dried to obtain 9.00 g of brown solid compound II, with a reaction yield of 71.04%.
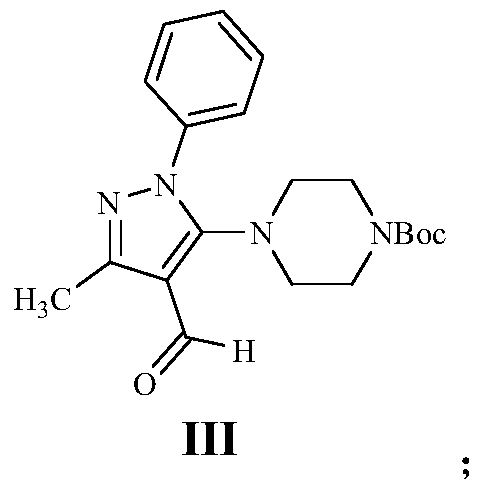
[0055](2) Dissolve 5.00g of compound II in N,N-dimethylformamide, add 6.81g of tert-butylpiperazine N-formate and 6.36g of cesium carbonate, heat up to 90°C and stir for 3 hours, the reaction is complete to obtain a brown The turbid solution, after returning the reaction solution to room temperature, adjusted the pH to less than 5 with dilute hydrochloric acid in an ice bath, extracted with ethyl acetate ...
Embodiment 2
[0064] A kind of synthetic method for Geglitin dimer impurity, comprises the following steps:
[0065] (1) Dissolve 10.00g of edaravone in N,N-dimethylformamide, add 18.22g of phosphorus oxychloride dropwise at room temperature, stir at 60°C for 12 hours to obtain a light green solution, and spot the plate The completion of the reaction was monitored; the reaction solution was slowly poured into ice water to quench, a solid product was precipitated from the water, the water phase was removed by filtration, and the filter cake was vacuum-dried to obtain 9.08 g of brown solid compound II, with a reaction yield of 71.06%.
[0066] (2) Dissolve 6.00g of compound II in N,N-dimethylformamide, add 4.81g of tert-butylpiperazine N-formate and 8.36g of sodium hydroxide, raise the temperature to 100°C and stir for 15 hours, and the reaction is complete to obtain a Brown-gray turbid liquid. After returning the reaction liquid to room temperature, adjust the pH to less than 5 with dilute h...
Embodiment 3
[0074] A kind of synthetic method for Geglitin dimer impurity, comprises the following steps:
[0075] (1) Dissolve 10.00g of edaravone in N,N-dimethylformamide, add 16.24g of NCS dropwise at room temperature, stir at 70°C for 12 hours to obtain a brown solution, and spot the plate to monitor the completion of the reaction; The reaction solution was slowly poured into ice water to quench, and a solid product was precipitated from the water. The water phase was removed by filtration, and the filter cake was vacuum-dried to obtain 8.57 g of brown solid compound II, with a reaction yield of 67.64%.
[0076] (2) Dissolve 8.00g of compound II in N,N-dimethylformamide, add 15.81g of tert-butylpiperazine N-formate and 20.36g of sodium carbonate, raise the temperature to 80°C and stir for 18 hours, the reaction is complete to obtain a brown The turbid solution, after returning the reaction solution to room temperature, adjusted the pH to less than 5 with dilute hydrochloric acid in an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com