Pharmaceutical co-crystal of gefitinib and bumetanide and preparation method thereof
A gefitinib and co-crystal technology, applied in the field of medicinal chemistry, can solve the problems of excessive organic solvent residues, poor reproducibility, low solubility, etc., to alleviate the toxic and side effects of drugs, improve chemical stability, and inhibit cancer cells. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
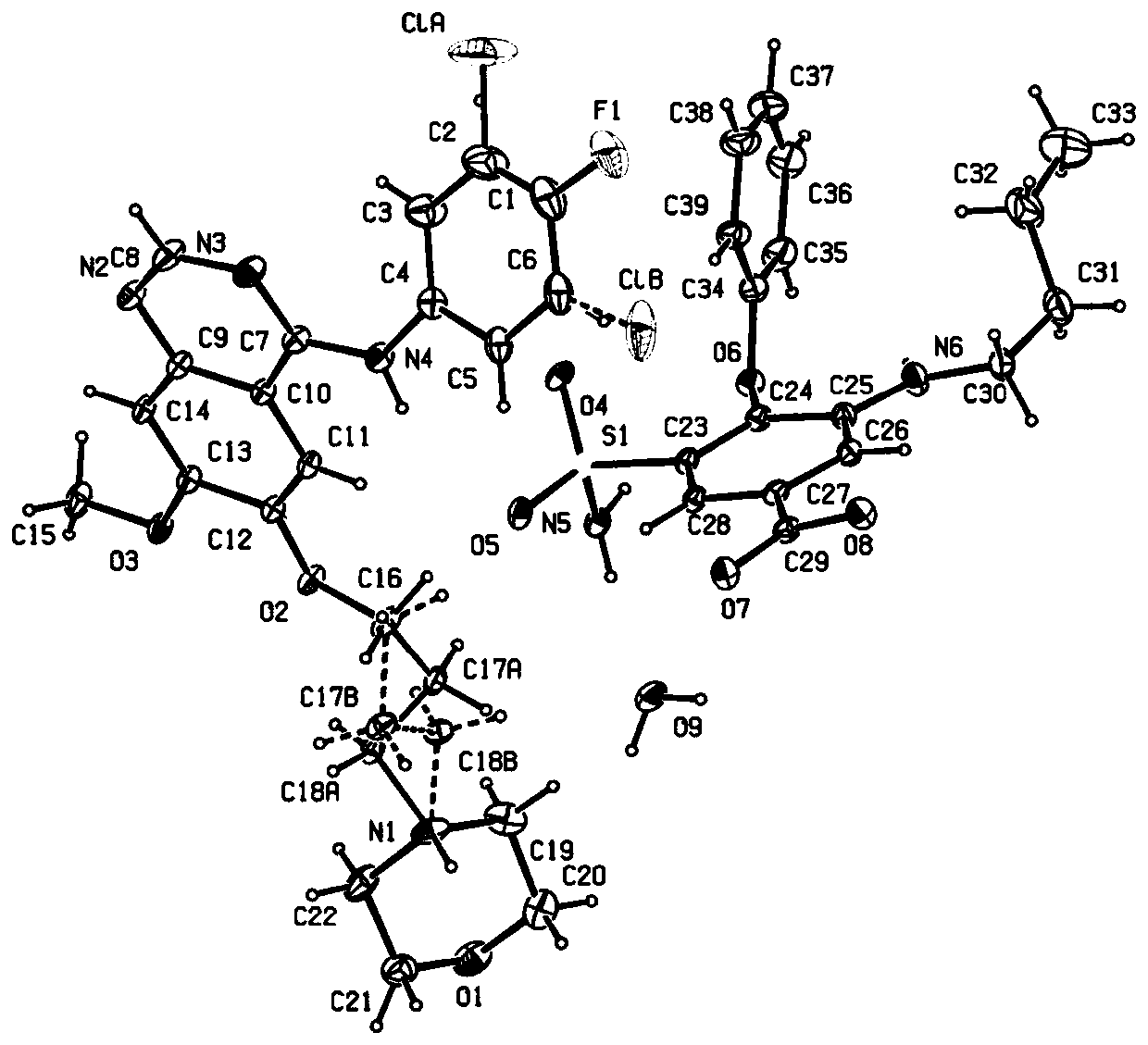
[0037] Preparation of gefitinib and bumetanide drug co-crystals: accurately weigh 89.4 mg of gefitinib and 72.3 mg of bumetanide in a 4ml centrifuge tube, mix in a vortex mixer for 10 min, and place the mixed sample Put it into a mortar, add 1mL of ethanol dropwise and grind it to a white powder, then add 10ml of ethanol, transfer the solution to a 25ml beaker, heat it in a water bath at 70°C for 10min to fully dissolve, and filter the solution through a microporous membrane with a pore size of 2.5μm Filtrate, cultivate the filtrate and let the solvent volatilize to precipitate crystals for 72 hours, filter and dry to obtain a bulk transparent crystal product that meets the requirements of single crystal diffraction, and perform single crystal diffraction experiments to determine its crystal structure.
[0038] The gefitinib and bumetanide co-crystals were tested using SuperNova, Dual, Cu athome / near, AtlasS2 diffractometer from Agilent Technologies, and the crystal testing tem...
Embodiment 2
[0052] Preparation of gefitinib and bumetanide drug co-crystals: accurately weigh 89.4 mg of gefitinib and 72.3 mg of bumetanide in a 4ml centrifuge tube, mix in a vortex mixer for 10 min, and place the mixed sample Put it into a mortar, drop 1mL of methanol and grind it to white powder, then add 10ml of methanol, transfer the solution to a 25ml beaker, heat it in a water bath at 70°C for 10min to fully dissolve, and pass the solution through a microporous filter membrane with a pore size of 2.5μm Filtrate, cultivate the filtrate and let the solvent volatilize to precipitate crystals for 72 hours, filter and dry to obtain irregular crystal products.
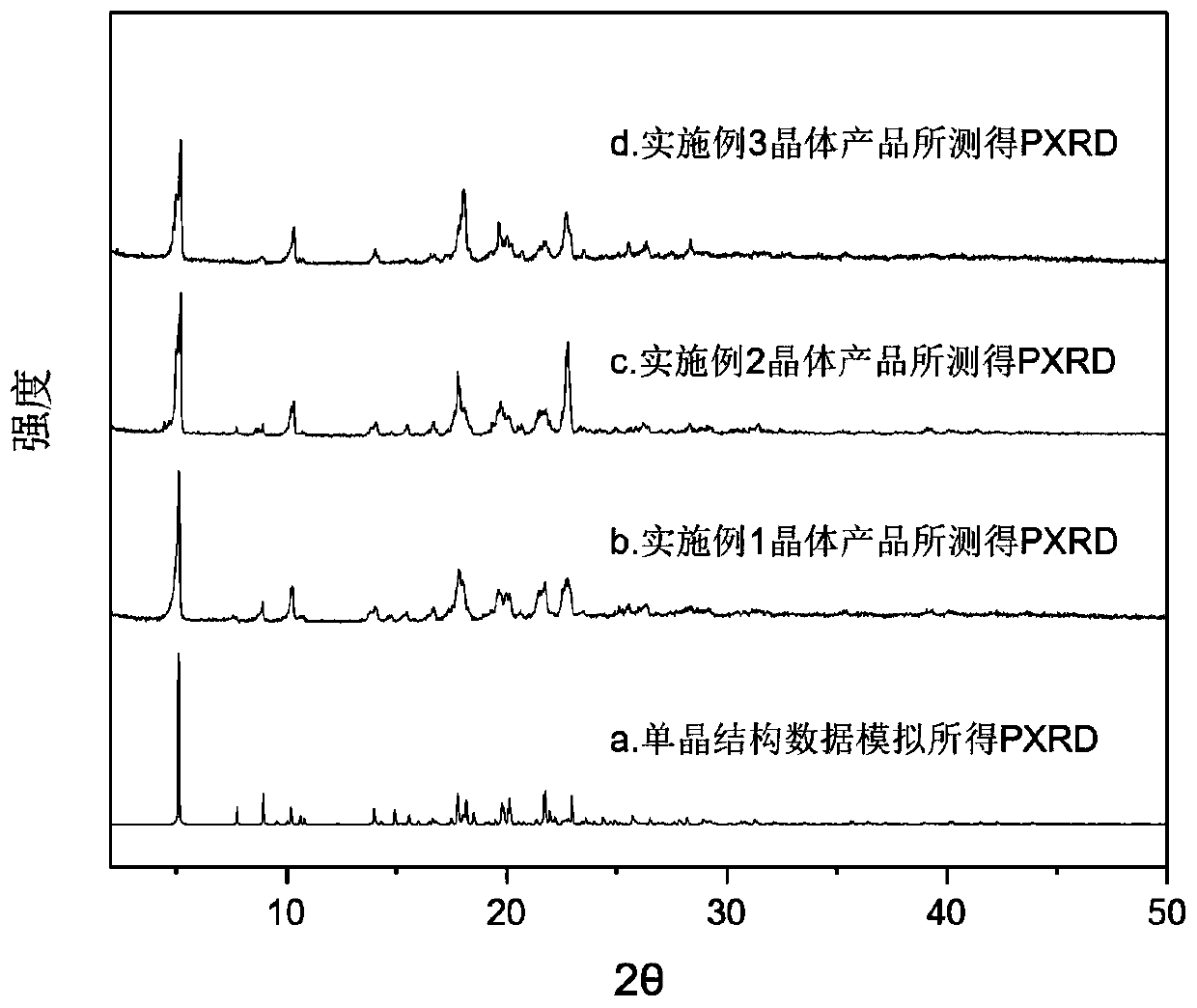
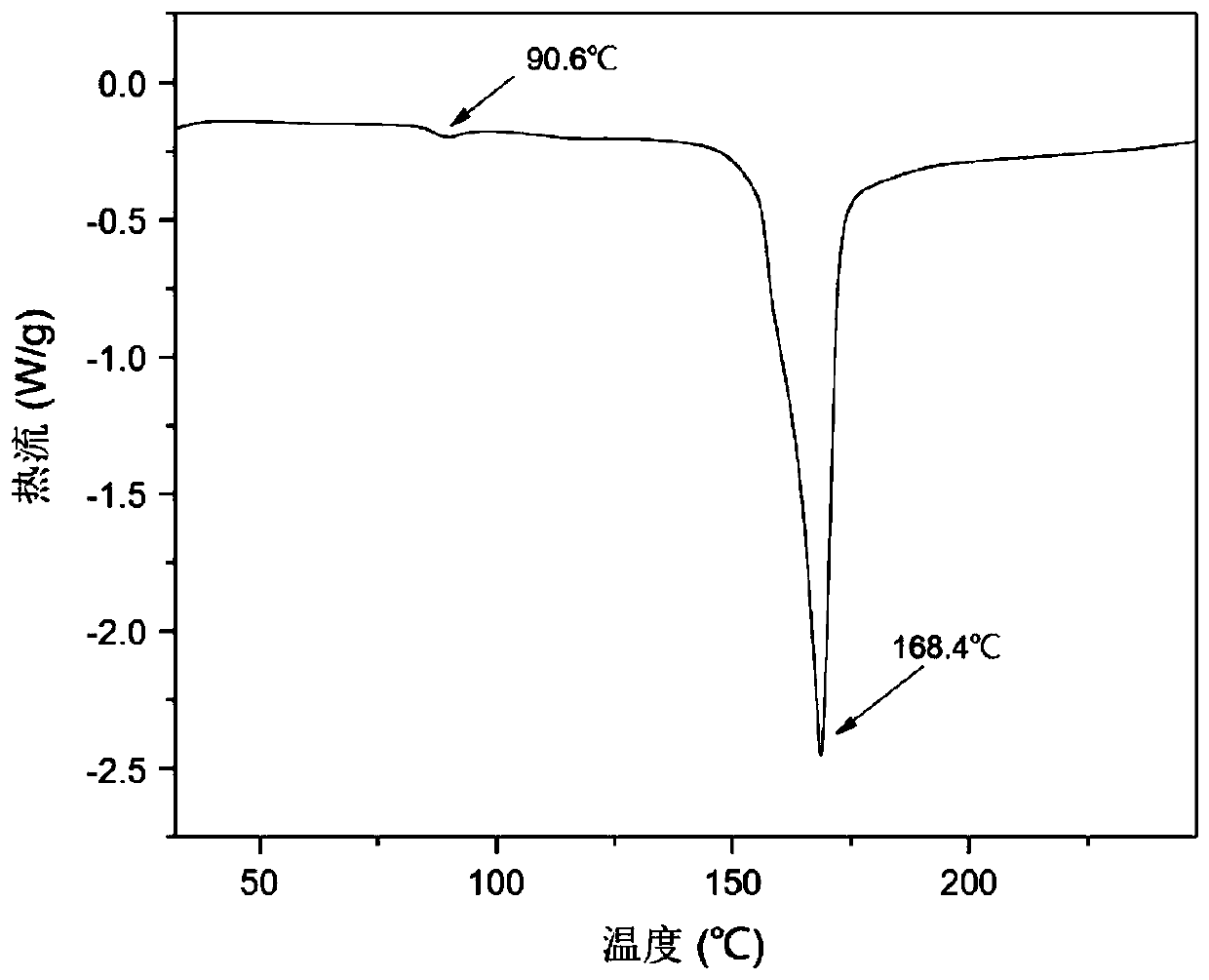
[0053] Such as figure 2 In the shown PXRD spectrogram, the positions of the PXRD diffraction peaks obtained in Example 2 and Example 1 are basically consistent and show that the crystal products obtained in Example 2 and Example 1 are the same eutectic, as Figure 4 The DSC spectral features shown as well as Figure 5 The show...
Embodiment 3
[0055] Preparation of gefitinib and bumetanide drug co-crystals: accurately weigh 89.4 mg of gefitinib and 72.3 mg of bumetanide in a 4ml centrifuge tube, mix in a vortex mixer for 10 min, and place the mixed sample Put it into a mortar, drop 1mL of methanol and grind it to white powder, then add 10ml of methanol, transfer the solution to a 25ml beaker, heat it in a water bath at 70°C for 10min to fully dissolve, and pass the solution through a microporous filter membrane with a pore size of 2.5μm Filtrate, cultivate the filtrate and let the solvent volatilize to precipitate crystals for 72 hours, filter and dry to obtain irregular crystal products.
[0056] Such as figure 2 In the shown PXRD spectrogram, the positions of the PXRD diffraction peaks obtained in embodiment 3 and embodiment 1 are basically consistent and show that the crystal products obtained in embodiment 3 and embodiment 1 are the same eutectic, as Figure 4 The DSC spectrogram features shown as well as Fi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com