Photochromic titanyl oxalate salt material and preparation method thereof
A titanyl oxalate, photochromic technology, applied in the direction of color-changing fluorescent materials, chemical instruments and methods, etc., can solve the problems of practical application limitations, inability to maintain photochromic properties, shortened life, etc. The effect of excellent reversible photochromic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] The invention provides a photochromic titanyl oxalate material and a preparation method thereof. The preparation method includes the following steps:
[0047] S1: Add a certain amount of potassium titanium oxalate (K 2 TiO·(C 2 O 4 ) 2 ), after stirring, a potassium titanium oxalate solution with a concentration of 0.05 to 0.35 M is obtained;
[0048] S2: adding hydrochloric acid with a concentration of 36 wt%-38 wt% to the potassium titanium oxalate solution obtained in step S1, and stirring uniformly to obtain a precursor solution, and subjecting the precursor solution to hydrothermal treatment to obtain a reaction product;
[0049] S3: Centrifugally separate the reaction product obtained in step S2, and dry it at 60-80° C. for 3-5 h after washing to prepare a photochromic titanium oxalate material.
[0050] In the following description, steps S1 to S3 will be described in detail.
[0051] In the present invention, step S1 is specifically: adding potassium titanium oxalate (K 2...
Embodiment 1
[0066] Add 0.53 g of potassium titanium oxalate to 30 mL of deionized water with pH=6, and heat and stir at 45°C for 10 minutes until the potassium titanium oxalate is completely dissolved to obtain a potassium titanium oxalate solution; stop stirring and wait until the potassium titanium oxalate solution is naturally cooled to After 25°C, quickly add 3.0 mL of hydrochloric acid to the potassium titanium oxalate solution, and stir at 25°C for 15 minutes until the hydrochloric acid and potassium titanium oxalate solution are evenly mixed as the precursor solution; transfer the precursor solution to an 80 mL reactor and seal it After that, it was placed in an oven and hydrothermally reacted at 110°C for 12 hours to obtain the reaction product; the reaction product was centrifuged, washed and dried at 60°C for 3 hours to obtain the photochromic titanium oxalate oxalate material with a particle size of 8μm .
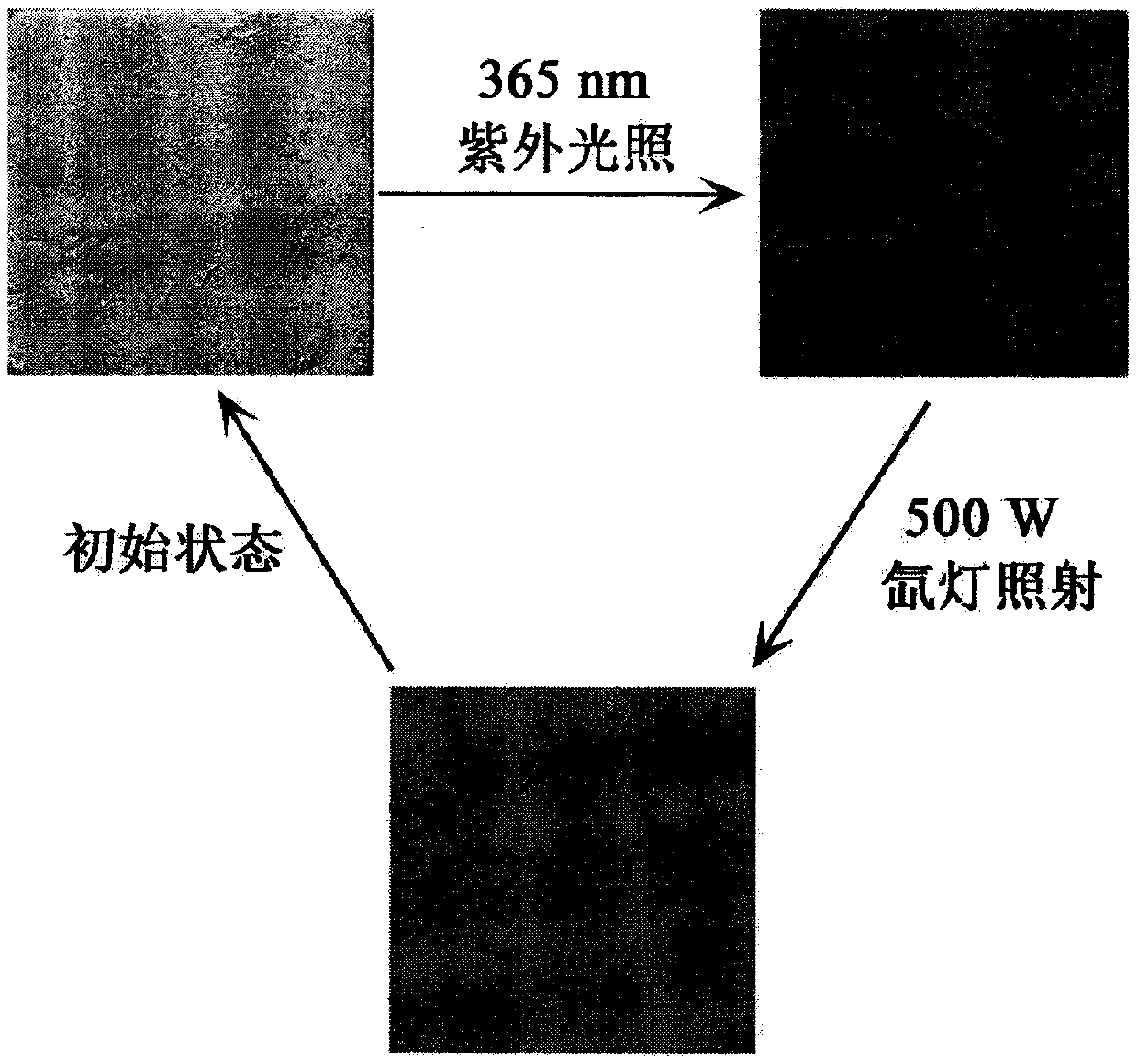
[0067] See figure 1 with figure 2 Shown is an SEM image of the photochrom...
Embodiment 2
[0071] Add 2.12g of potassium titanium oxalate to 30mL of deionized water with pH=6, heat and stir at 45°C for 10 minutes, until potassium titanium oxalate is completely dissolved, and obtain potassium titanium oxalate solution; stop stirring and wait until the potassium titanium oxalate solution is naturally cooled to 25 After ℃, quickly add 3.0 mL of hydrochloric acid to the potassium titanium oxalate solution, stir at 25 ℃ for 15 minutes, until the hydrochloric acid and potassium titanium oxalate solution are evenly mixed, as the precursor solution; transfer the precursor solution to an 80 mL reactor, and airtight After that, it was placed in an oven and hydrothermally reacted at 100°C for 12 hours to obtain the reaction product; the reaction product was centrifuged, washed and dried at 60°C for 3 hours to obtain the photochromic oxalate titanyl oxalate material with a particle size of 10 μm .
[0072] See Figure 5 with Image 6 Shown is the photochromic titanium oxalate mate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com