Molecular probe of higher plant non-cobalt-dependent methionine synthase and application of molecular probe
A technology of methionine synthase and higher plants, applied in the field of highly selective probes, can solve problems such as unclear binding of probes, and achieve the effect of high specificity and high affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0046] Biological experiments
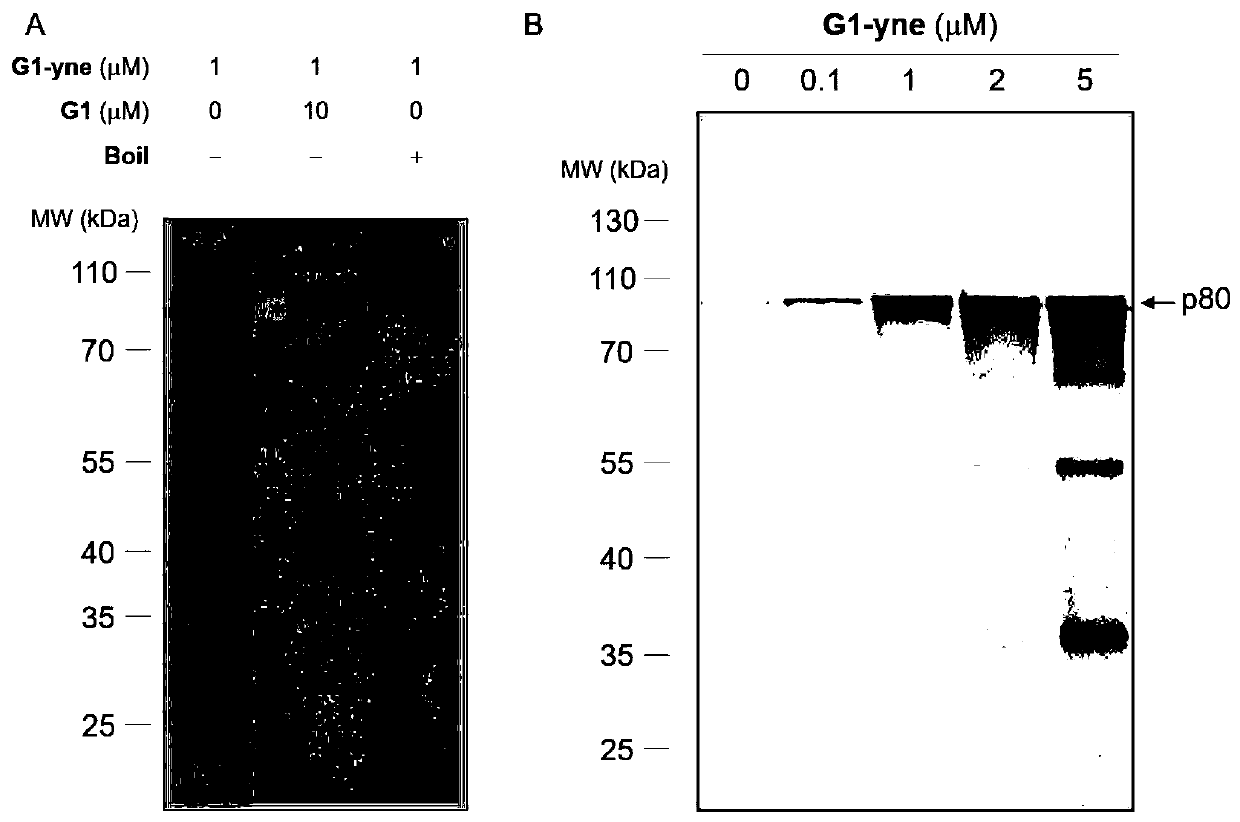
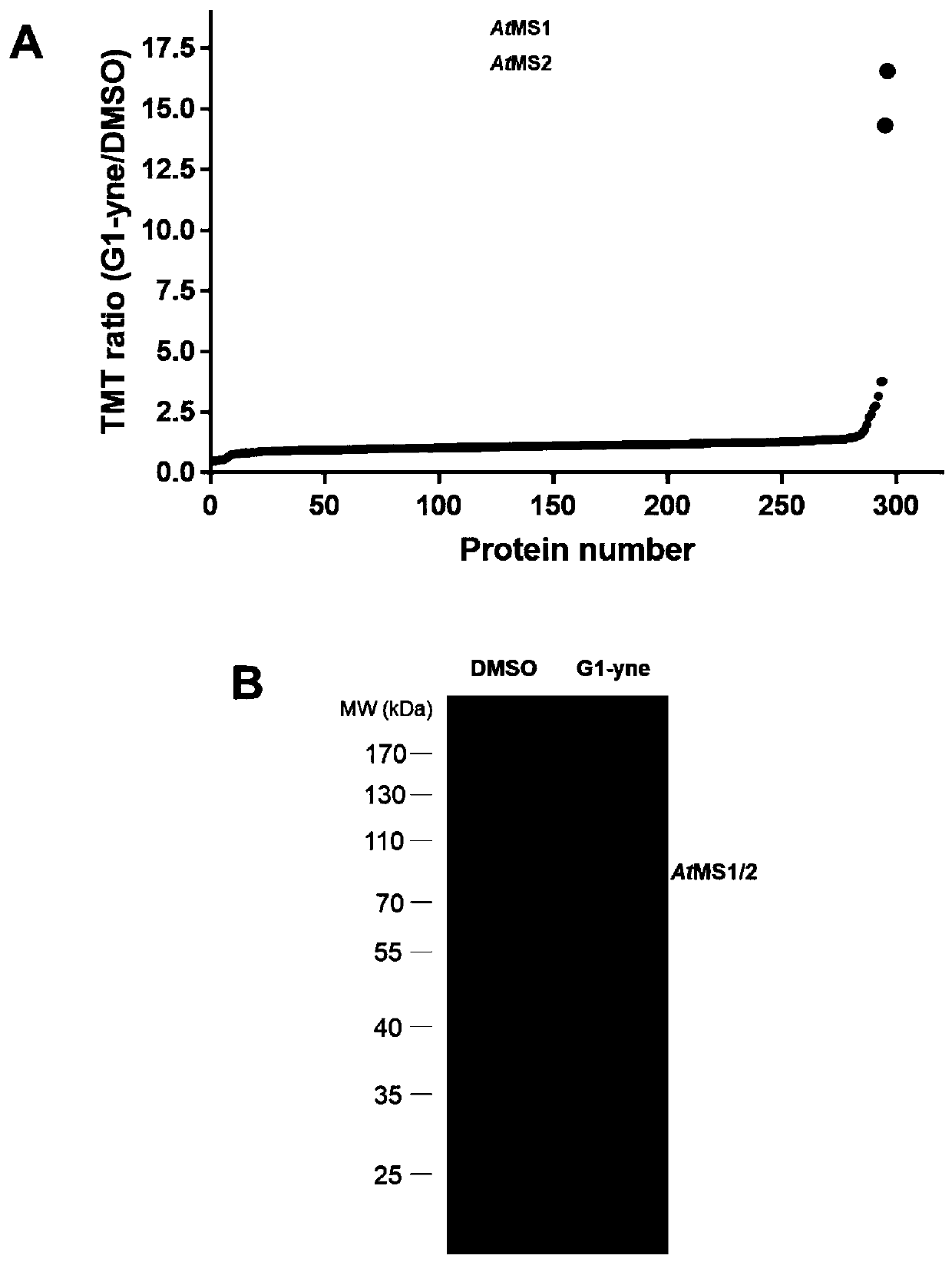
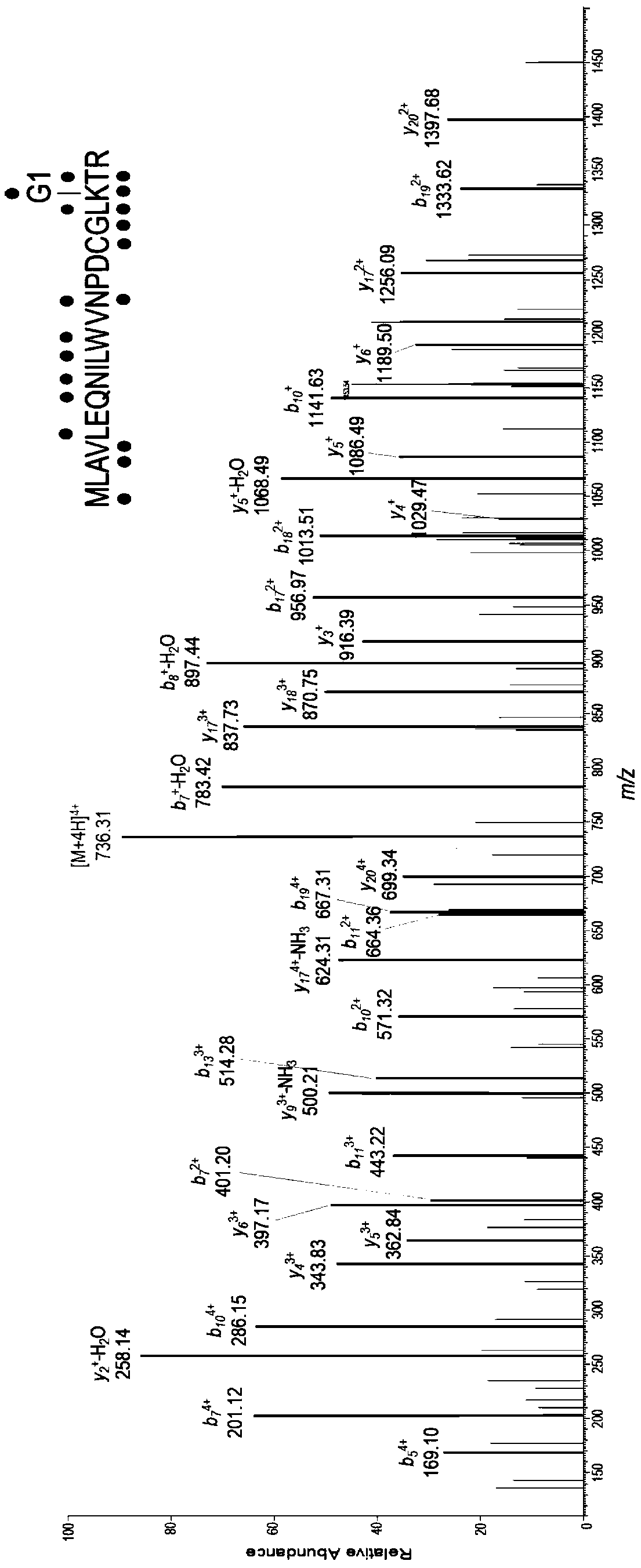
[0047] Protein labeling of Arabidopsis tissue crude extracts based on gel electrophoresis fluorescence scanning.
[0048] In reaction buffer (50mM HEPES, pH 7.4, 150mM NaCl, 5mM MgCl 2) to a final concentration of 2.5 μg / μL and add a final concentration of 1 μM probe G1-yne to incubate for 1 hour at room temperature. Then add SDS at a final concentration of 1% to the protein sample and perform a "click" reaction: For each reaction, add 0.2 μL of TAMRA-N to 19.2 μL of the protein sample 3 (10mM stock solution in DMSO, Lumiprobe), CuSO 4 (100 mM stock in water), THPTA (10 mM stock in water, Sigma) and sodium ascorbate (100 mM stock in water). The samples were incubated at room temperature in the dark for 1 hour, and the reaction was terminated by adding 5 μL of 5×SDS loading buffer and boiling at 95° C. for 15 minutes. Samples were applied to 10% Bis-tris denaturing gels and in-gel fluorescence scanning was performed with a FUJIFILM FLA 9000 p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com