Liver cancer diagnosis and treatment marker, namely LINC02128
A liver cancer and liver cancer treatment technology, applied in the field of biomedicine, can solve problems such as functions that need to be further clarified, and achieve the effect of improving accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
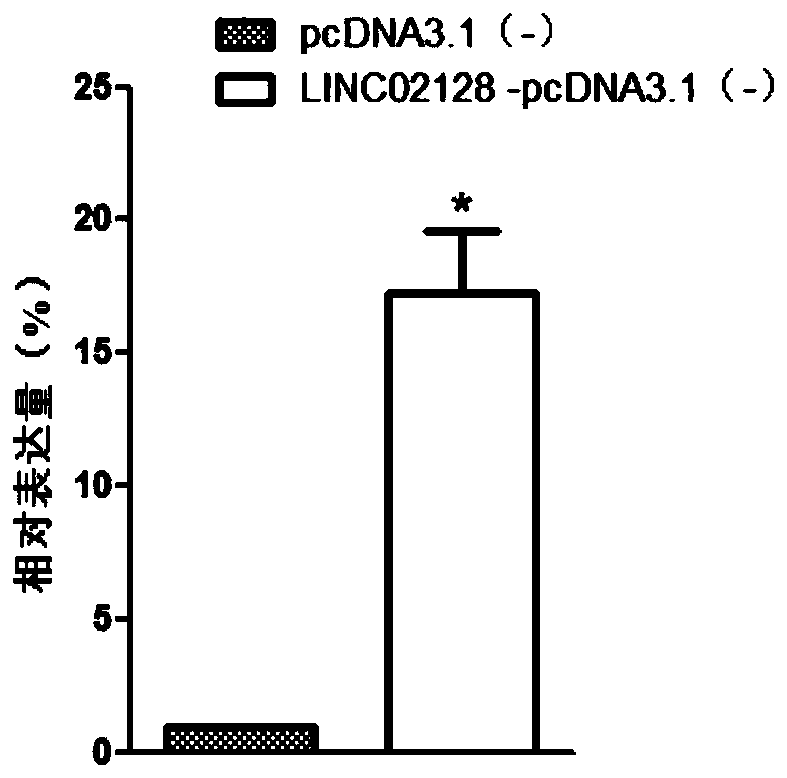
[0062] Example 1 QPCR detection of differential expression of LINC02128
[0063] 1. RNA extraction
[0064] Tissue RNA was extracted using QIAGEN tissue RNA extraction kit, and the specific steps in the instructions were followed.
[0065] 2. Reverse transcription reaction
[0066] Use 25μl reaction system, take 1μg total RNA for each sample as template RNA, and add the following components to PCR tubes: DEPC water, 5× reverse transcription buffer, 10mM dNTP, 0.1mM DTT, 30μM Oligo dT, 200U / μl M-MLV, template RNA. Incubate at 42°C for 1 hour, then centrifuge briefly at 72°C for 10 minutes.
[0067] 3. Real-time fluorescent quantitative PCR
[0068]PCR amplification was performed using cDNA as a template. The upstream primer of LINC02128 is 5′-CAGGAGCCAGAGAATCAT-3′ (SEQ ID NO.2), the downstream primer is 5′-TCACGGAGGTTTACCAAAT-3′ (SEQ ID NO.3); GAPDH is an internal reference, and the upstream primer is 5′-CCGGGAAACTGTGGCGTGATGG-3′ (SEQ ID NO.4), the downstream primer is 5'...
Embodiment 2
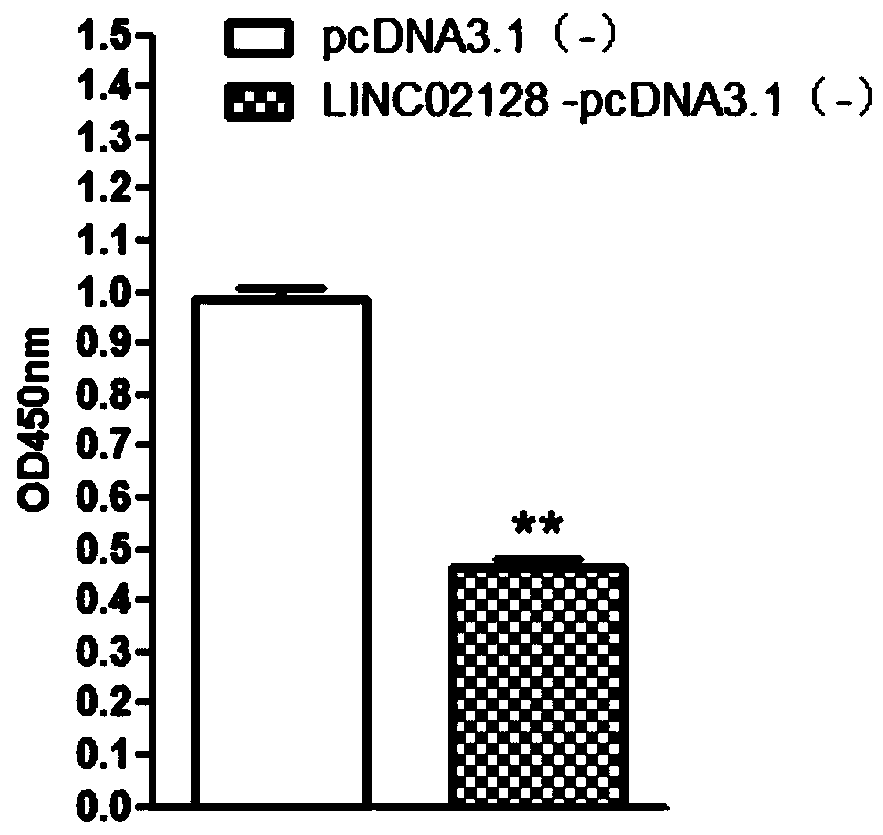
[0073] Example 2 Effect of LINC02128 gene on the proliferation of liver cancer cells
[0074] 1. Cell culture and cell transfection
[0075] Construct the LINC02128 overexpression vector according to conventional techniques, and the general steps are as follows: amplify LINC02128 to obtain the cDNA sequence, insert it into the eukaryotic expression vector pcDNA3.1(-), construct the LINC02128-pcDNA3.1(-) recombinant vector, and insert It transforms competent cells, picks clones, and double-enzyme cuts identification to obtain LINC02128-pcDNA3.1(-) expression vector containing complete and correct LINC02128 sequence. Plasmids were prepared in large quantities using an endotoxin-free kit.
[0076] Human hepatoma cell SMMC-7721 cells were inoculated in DMEM medium containing 10% fetal bovine serum in 5% CO 2 , Cultured in a constant temperature incubator at 37°C. 24h before transfection press 3×10 5 Inoculate each well in a 6-well plate, and add 2 mL of serum-free medium to ma...
Embodiment 3
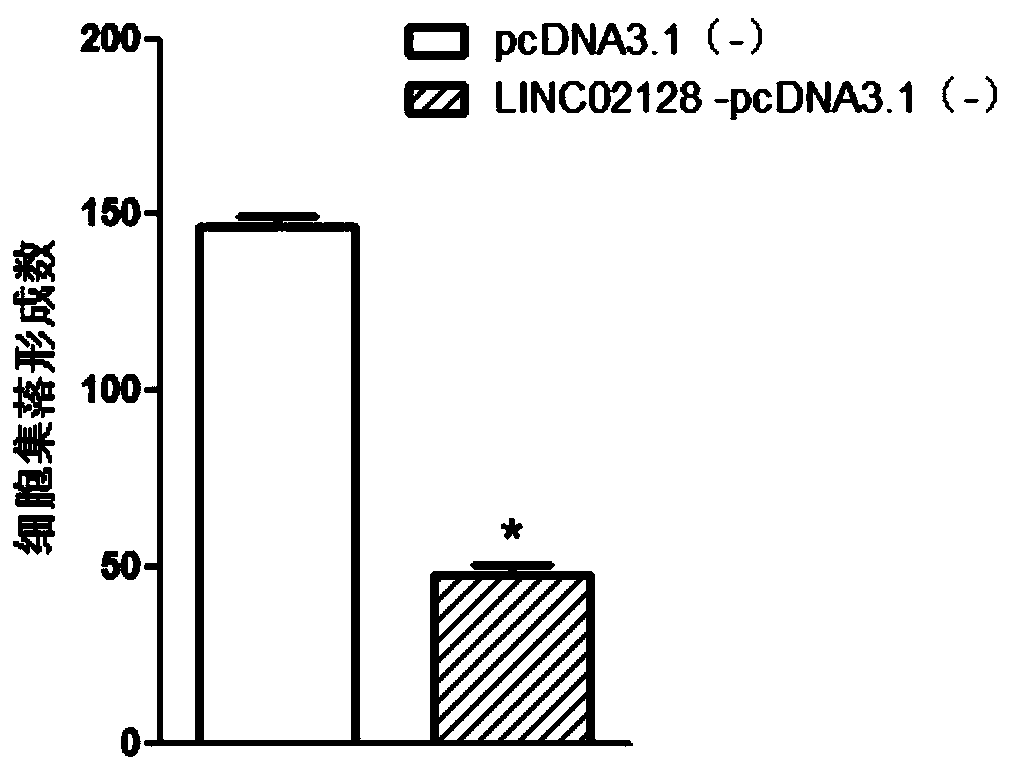
[0083] Example 3 Soft agar colony formation experiment
[0084] 1. Experimental steps
[0085] (1) Digest the cells with 0.25% trypsin, pipette gently to make a single cell suspension, and collect the cell pellet by centrifugation.
[0086] (2) Resuspend with DMEM complete medium containing 20% fetal bovine serum, count after appropriate dilution, and adjust the cell concentration to 5×10 3 pieces / ml.
[0087] (3) Prepare two low-melting-point agarose solutions with concentrations of 1.2% and 0.7%, respectively, and maintain them in a 40° C. water bath after autoclaving.
[0088] (4) Mix 1.2% agarose and 2×DMEM medium 1:1, add 2×antibiotics and 20% calf serum, take 3ml of the mixed solution and inject it into a 6cm-diameter plate, let it cool and solidify for 5 minutes, and place it as the bottom agar CO 2 Reserve in the incubator.
[0089] (5) Mix 0.7% agarose and 2×DMEM medium 1:1 in a sterile test tube, then add 0.2ml to the tube with a concentration of 5×10 3 cells...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com