Non-woven fabric material and application thereof
A technology of non-woven fabrics and reaction temperature, applied in the direction of non-woven fabrics, textiles and papermaking, cellulose/protein conjugated artificial filaments, etc. Anti-cross-color effect and other issues to achieve the effect of ensuring the use effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] One, the preparation of modified cellulose A
[0040] One), the preparation of (4-vinylphenyl-4'-methylenecarboxyphenyl) iodonium
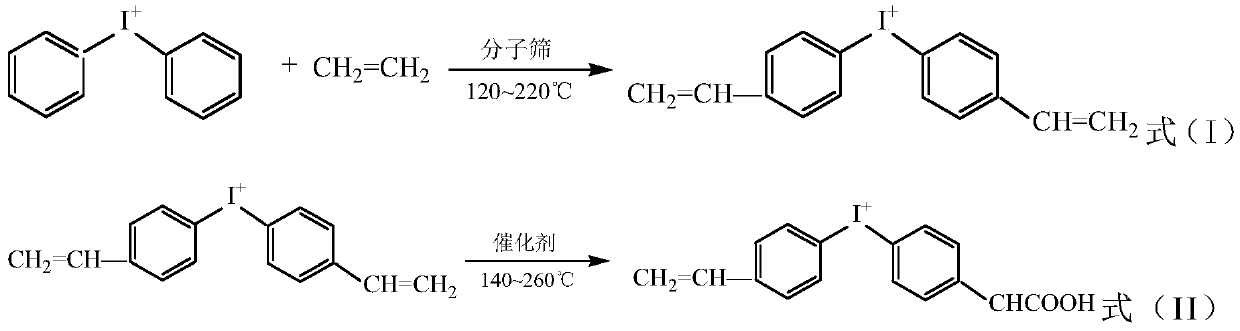
[0041] 1. Using molecular sieves as a catalyst, diphenyliodonium salt reacts with ethylene to obtain 4,4'-divinyldiphenyliodonium (as shown in formula (I)) (embodiment 1-1-1, 1 The reaction conditions of -1-2 and 1-1-3 are shown in Table 1), 4,4'-divinyldiphenyliodonium is partially oxidized through the supported palladium (Pd) heteropolyacid catalyst system Obtain 4-vinylphenyl-4'-methylenecarboxyphenyl) iodonium (as shown in formula (II)) (embodiment 1-1-1, 1-1-2, 1-1-3 reaction The conditions are shown in Table 2):
[0042]
[0043] Among them, the molecular sieve is sodium ZSM-5 molecular sieve exchanged by magnesium, calcium or barium.
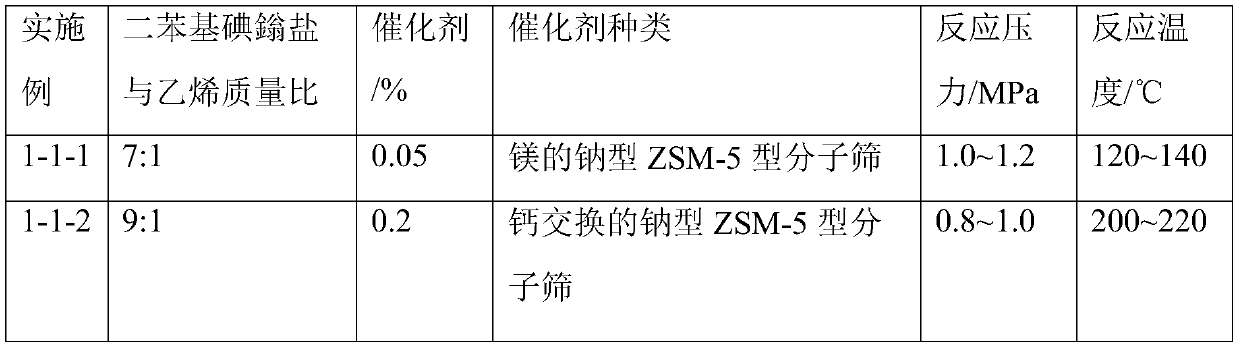
[0044] Table 1 Diphenyliodonium salt and ethylene reaction conditions and substance consumption
[0045]
[0046]
[0047] Table 2 Reaction conditions and amounts of substances used in par...
Embodiment 1-2-1
[0051] 1. Synthesis of (4-vinylphenyl-4'-methylenecarboxyphenyl)iodonium-terminated polyvinylpyrrolidone
[0052] The monomer vinylpyrrolidone (NVP) is configured into an aqueous solution with a mass fraction of 45%, using a small amount of hydrogen peroxide (0.2% of NVP) as a catalyst, under the action of azobisisobutyronitrile (0.3% of NVP), in Initiate polymerization at 40-50°C, react for 2 hours, and the monomer polymerization conversion rate is 93-95%. Add (4-vinylphenyl-4'-methylenecarboxyphenyl) iodonium (amount of 0.5% NVP) prepared in Example 1-1-1, react for 0.2 hours, and seal the polymer terminal to obtain (4-vinylphenyl-4'-methylenecarboxyphenyl)iodonium-terminated polyvinylpyrrolidone.
[0053] Then add 0.2% ammonia water to the polymer to decompose the remaining azobisisobutyronitrile. The polymer is spray-dried under hot air at 120-135°C to obtain powdery (4-vinylphenyl-4'-methylenecarboxyphenyl)iodonium-terminated polyvinylpyrrolidone. (4-vinylphenyl-4'-m...
Embodiment 1-2-2
[0065] 1. Synthesis of (4-vinylphenyl-4'-methylenecarboxyphenyl)iodonium-terminated polyvinylpyrrolidone
[0066] The monomer vinylpyrrolidone (NVP) was configured into an aqueous solution with a mass fraction of 50%. Use a small amount of hydrogen peroxide (0.3% NVP) as a catalyst, under the action of azobisisobutyronitrile (0.4% NVP), initiate polymerization at 50-60°C, react for 3 hours, and the monomer polymerization conversion rate is 96~98%.Add ((4-vinylphenyl-4-methylenecarboxyphenyl) iodonium (consumption is the NVP of 0.8%) that embodiment 1-1-1 makes, reacted 0.5 hour, Capping the polymer yields (4-vinylphenyl-4-methylenecarboxyphenyl)iodonium-capped polyvinylpyrrolidone.
[0067] Add 0.3% ammonia water to the polymer to decompose the remaining azobisisobutyronitrile. The polymer is spray-dried under hot air at 135-150°C to obtain powdery (4-vinylphenyl-4-methylenecarboxyphenyl)iodonium-terminated polyvinylpyrrolidone. (4-vinylphenyl-4'-methylenecarboxyphenyl) i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com