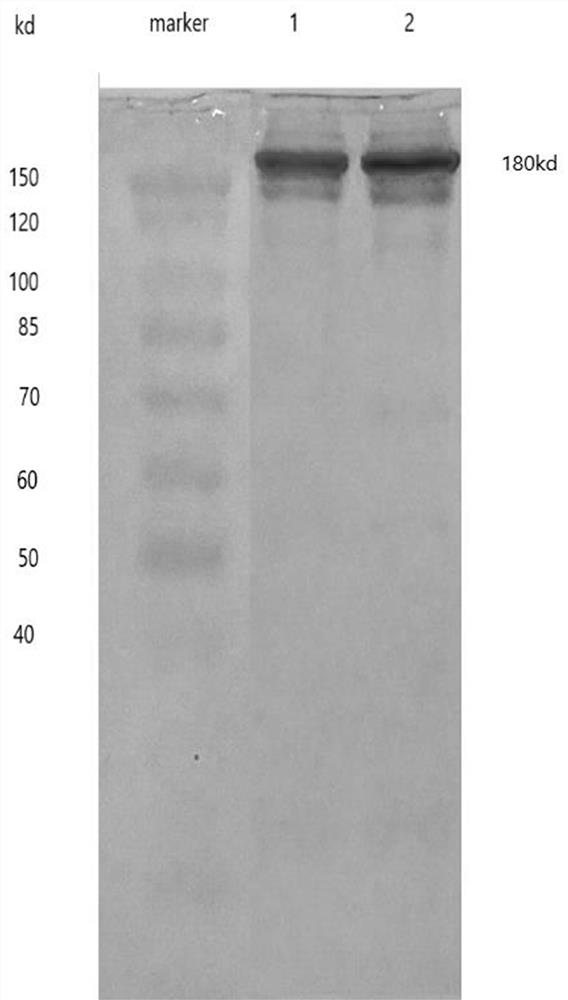
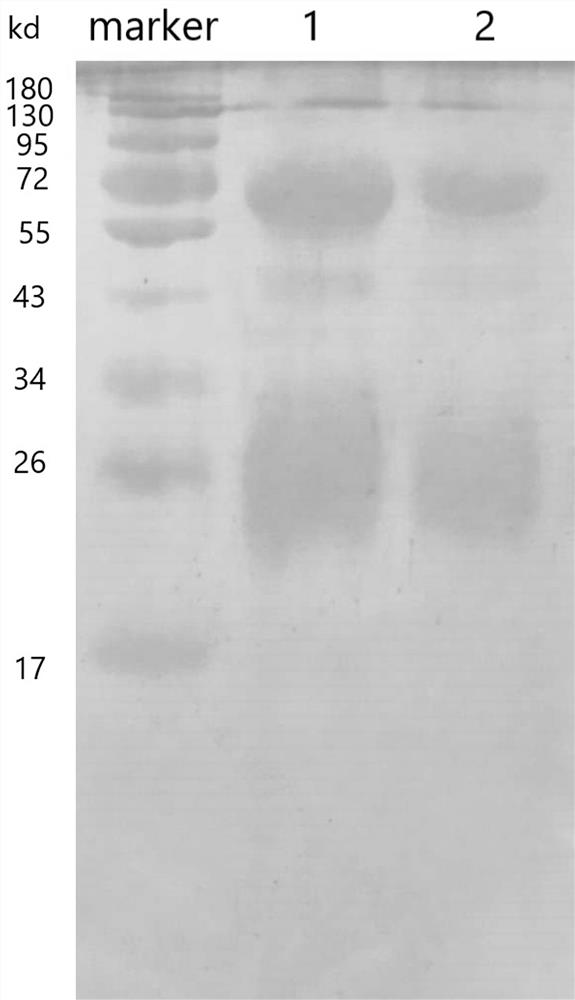
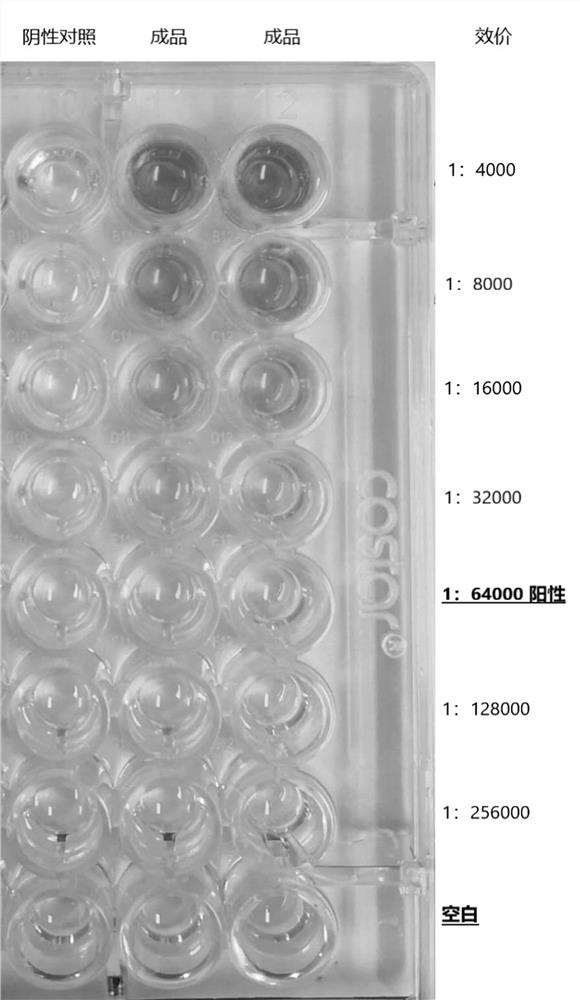
A kind of anti-new coronavirus antibody and its preparation method and application
A coronavirus and a new type of technology, applied in the field of anti-new coronavirus antibodies and their preparation, can solve the problems of limited auxiliary role in group virus prevention and control, inability to achieve specific killing of pathogens, and high degree of dependence on the external environment, which is not easy to achieve. Allergy, wide range of protection, low-dose effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The present invention specifically provides an anti-new coronavirus antibody, which is characterized in that the antibody is a high-titer specific antibody extracted from chicken egg yolk, and the chicken egg yolk is mainly obtained from eggs produced by immunized hens. Obtained, the immunized hen is a healthy hen injected with a new coronavirus vaccine.
[0029] Wherein, the novel coronavirus vaccine is mainly prepared from recombinantly expressed novel coronavirus (SARS-CoV-2) S antigen or virus-inactivated novel coronavirus (SARS-CoV-2) antigen.
[0030] Its specific preparation method comprises the following steps:
[0031] 1. Antibody preparation:
[0032] S1. Vaccine preparation:
[0033] Take the recombinantly expressed new coronavirus (SARS-CoV-2) S antigen or the virus-inactivated new coronavirus (SARS-CoV-2) antigen, and add the antigen to Freund's complete adjuvant at a ratio of 1:1 (initial immunization) or Freund's incomplete adjuvant (boosting immunizat...
Embodiment 2
[0056] This embodiment compares the yolk antibody production situation of two groups of different antigen immunization doses, and the preferred antigen immunization dose is specifically shown in the following table:
[0057] Table 1 The rise and fall of egg yolk antibody titers with different antigen immunization doses
[0058]
[0059] (NT: As the hen did not lay eggs after immunization, no detection was performed.)
[0060] It can be seen from the above table that the 5 μg / feather antigen dose group is preferred, and the yolk antibody titers produced in this dose group after 6, 8, and 10 weeks of primary vaccination are not lower than the 10 μg / feather antigen dose group.
Embodiment 3
[0062] In this embodiment, in order to verify the anti-new coronavirus yolk antibody neutralizing virus performance, the following tests were carried out:
[0063] Take 105~107 TCID50 / ml of the new coronavirus liquid and mix them with the new coronavirus egg yolk antibody samples with concentrations of 1mg / ml, 2mg / ml, and 3mg / ml according to the volume ratio of 1:1, and mix them evenly, and neutralize at 25~37℃ for 1 After ~2 hours, add it to a 96-well plate containing cells, incubate in a carbon dioxide incubator at 33-35°C, and observe cell changes daily under a microscope.
[0064] Under the condition of 25-37 ℃, the concentration of 2mg / ml and 3mg / ml of the new coronavirus egg yolk antibody was mixed with the new coronavirus liquid of 105~107TCID50 / ml, and the cells had no pathological changes. As a positive control, the novel coronavirus liquid can cause pathological changes in cells.
[0065] It can be seen from the test results that the anti-new coronavirus IgY prepare...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com