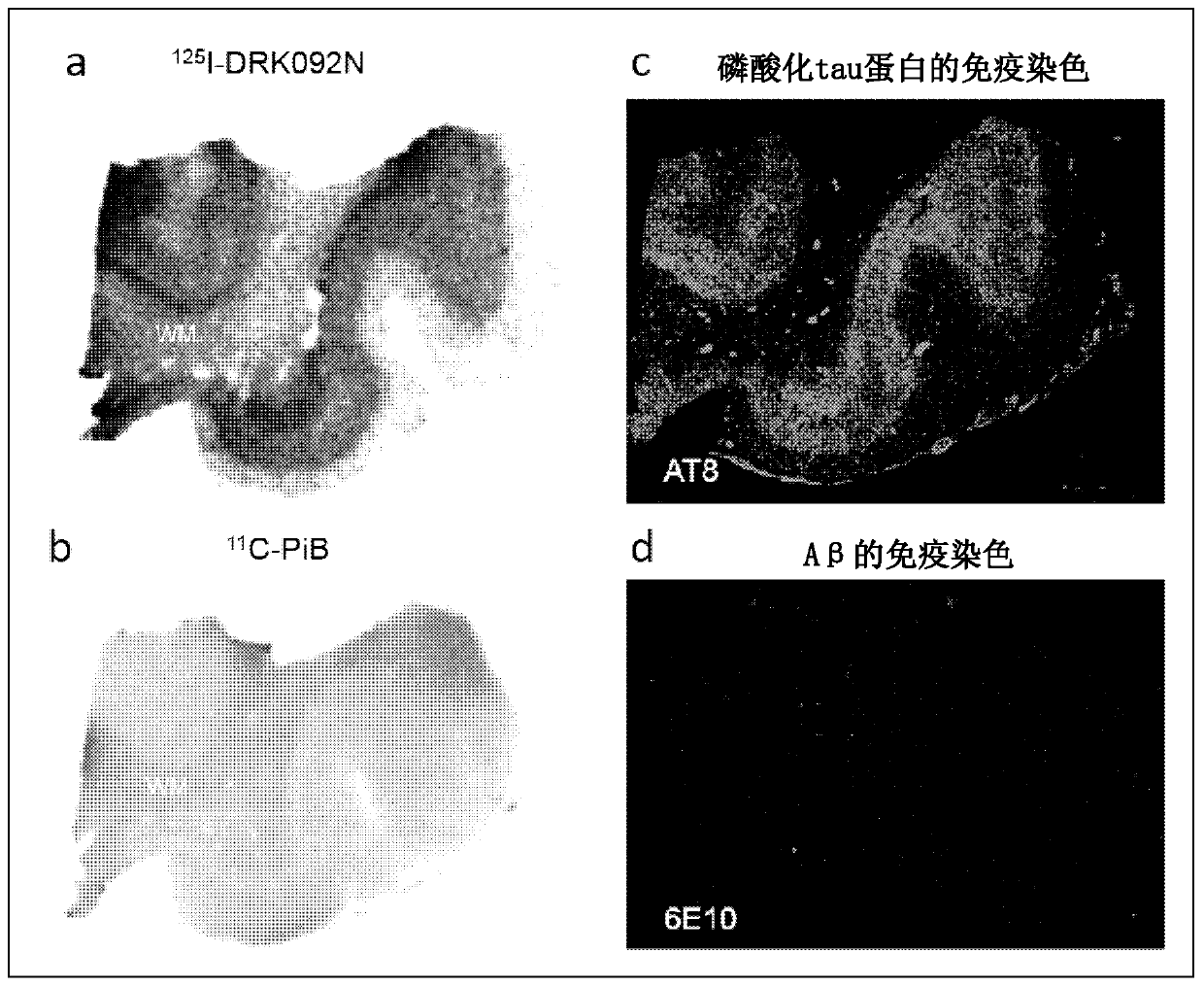
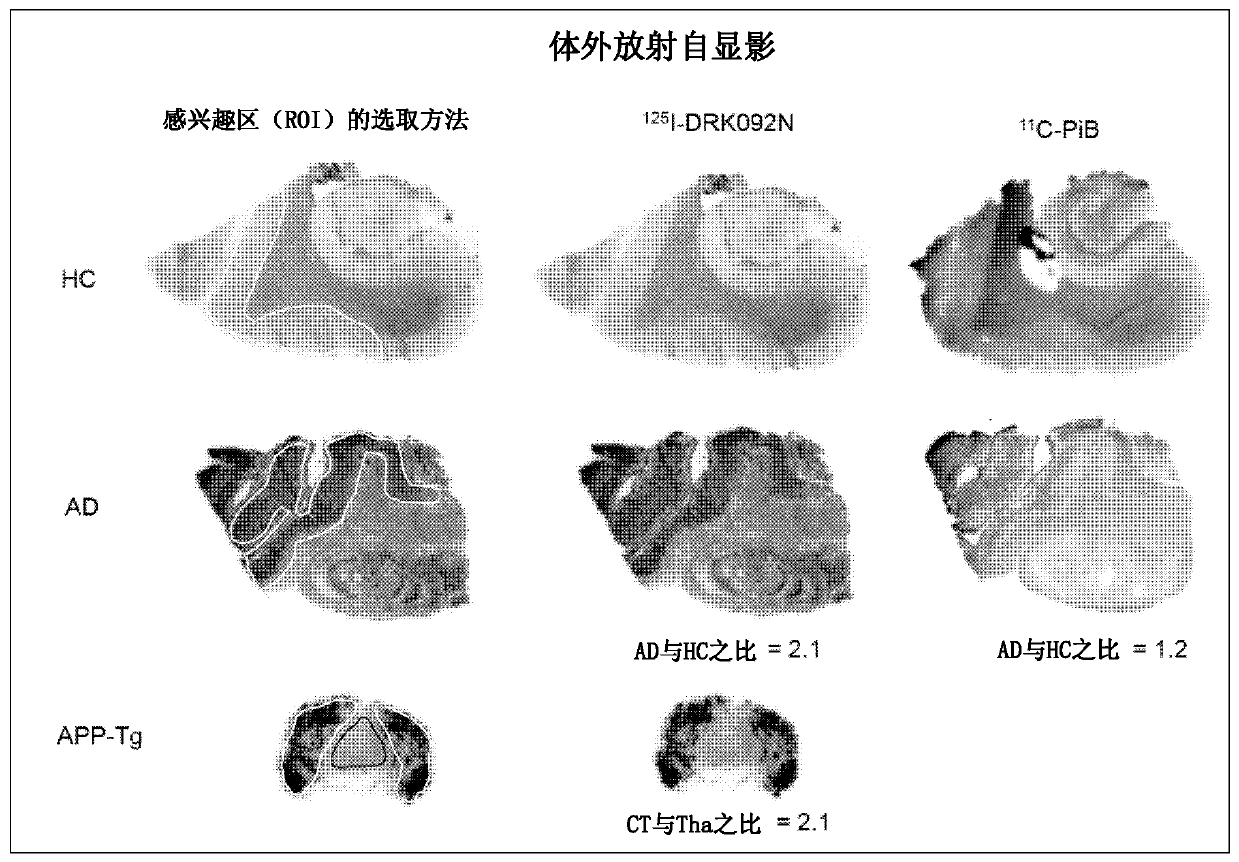
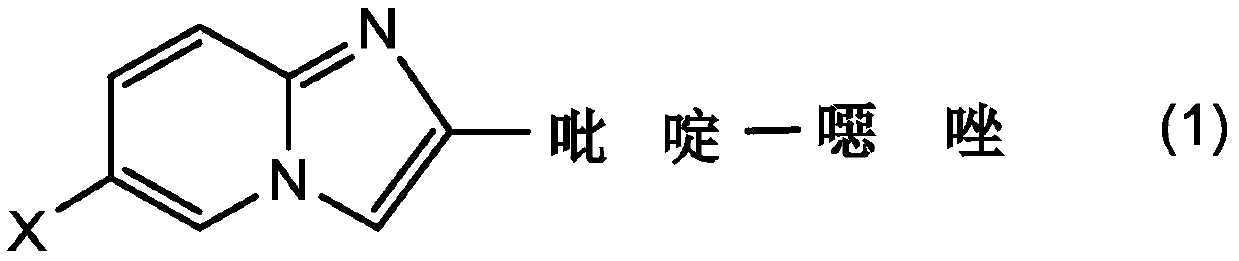
Radionuclide-labeled compound and imaging agent containing same
A technology of radionuclides and compounds, applied in the directions of in vivo radioactive preparations, radioactive carriers, isotope introduction of heterocyclic compounds, etc., can solve the problems of inability to image amyloid and tau protein at the same time and high affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1: Synthesis of DRK092N Labeled Precursor (Compound 10)
[0083] Compound 10 was synthesized according to the scheme shown below.
[0084]
Embodiment 1-1
[0085] Embodiment 1-1: Synthesis of Compound 2
[0086] Under an argon atmosphere, 848 mL of a diethyl ether solution of 50.9 g of Compound 1 was cooled, and 131 mL of a 1.64 mol / L n-BuLi hexane solution was added dropwise over 30 minutes at -62°C or lower. After stirring at this temperature for 30 minutes, 21.5 g of dehydrated N,N-dimethylacetamide was added dropwise over 10 minutes. Aqueous ammonium chloride solution was added and stirred at room temperature for 2 days. It extracted with ethyl acetate, dried the organic layer with anhydrous magnesium sulfate, and concentrated under reduced pressure. Compound 2 was used directly in the following reaction without further purification.
Embodiment 1-2
[0087] Embodiment 1-2: the synthesis of compound 3
[0088]40.0 g of 1,2-ethanediol and 12.3 g of p-toluenesulfonic acid monohydrate were added to 830 mL of a toluene solution of compound 2, and the generated water was removed, followed by reflux for 20 hours. After cooling, an aqueous solution of sodium carbonate was added, and a small amount of ethyl acetate was added for extraction. The obtained organic layer was washed with water, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure.
[0089] The concentrated residue was purified by silica gel column chromatography (mobile phase: heptane / ethyl acetate=9 / 1 to 7 / 3) to obtain 24.3 g of Compound 3 (yield 46%).
[0090] 1 H-NMR (CDCl 3 )δ: 8.48 (1H, s), 7.63 (1H, dd, J = 8.2, 4.1Hz), 7.45 (1H, dd, J = 8.2, 4.1Hz), 4.08-4.05 (2H, m), 3.81-3.75 (2H, m), 1.67 (3H, s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com