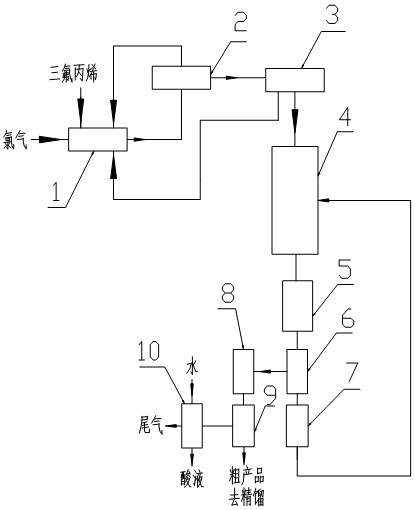
A kind of preparation method of 2-chloro-3,3,3-trifluoropropene
A technology of trifluoropropene and trifluoropropane, which is applied in the field of preparation of 2-chloro-3,3,3-trifluoropropene, can solve the problem that the inhibition of catalyst deactivation cannot be well achieved, the requirement of HF water content is high, and the product Problems such as low selectivity, to achieve the effect of being conducive to product diffusion, reducing product coking, and simple process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The photochlorination reaction kettle is equipped with a light source of partial ultraviolet wavelength and 2,3-dichloro-1,1,1-trifluoropropane. The feed flow rate of the 2,3-dichloro-1,1,1-trifluoropropane absorption liquid at the top of the rectification tower to the light chlorination reactor is 10mol / h, and the 3,3,3-trifluoropropene is fed to the light chlorine The feed flow rate of the chemical reactor is 40.8mol / h, the feed flow rate of chlorine gas to the photochlorination reactor is 40mol / h, the reaction temperature is 45°C, and the reaction residence time is 0.5h.
[0034] After the reaction is completed, the molar fraction of each component in the photochlorination reactor is: 2,3-dichloro-1,1,1-trifluoropropane 0.997, 2,3,3-trichloro-1,1,1-tri Fluoropropane 0.001, 3,3,3-trifluoropropene 0.002, use the material pump to transport the material in the photochlorination reactor to the rectification tower at a liquid phase flow rate of 50.8mol / h, and 3,3, The fee...
Embodiment 2
[0038]The photochlorination reaction kettle is equipped with a light source of partial ultraviolet wavelength and 2,3-dichloro-1,1,1-trifluoropropane. The feed flow rate of the 2,3-dichloro-1,1,1-trifluoropropane absorption liquid at the top of the rectification tower to the light chlorination reactor is 10mol / h, and the 3,3,3-trifluoropropene is fed to the light chlorine The feed flow rate of the chemical reactor is 40.8mol / h, the feed flow rate of chlorine gas to the photochlorination reactor is 40mol / h, the reaction temperature is 35°C, and the reaction residence time is 5h.
[0039] After the reaction is completed, the molar fraction of each component in the chlorination reactor is: 2,3-dichloro-1,1,1-trifluoropropane 0.995, 2,3,3-trichloro-1,1,1-trifluoro Propane 0.002, 3,3,3-trifluoropropene 0.003, use the material pump to transport the material in the photochlorination reactor to the rectification tower at a liquid phase flow rate of 50.8mol / h, and transfer the 3,3,3 -...
Embodiment 3
[0043] The photochlorination reaction kettle is equipped with a light source of partial ultraviolet wavelength and 2,3-dichloro-1,1,1-trifluoropropane. The feed flow rate of the 2,3-dichloro-1,1,1-trifluoropropane absorption liquid at the top of the rectification tower to the light chlorination reactor is 10mol / h, and the 3,3,3-trifluoropropene is fed to the light chlorine The feed flow rate of the chemical reactor is 40.8mol / h, the feed flow rate of chlorine gas to the photochlorination reactor is 40mol / h, the reaction temperature is 75°C, and the reaction residence time is 0.5h.
[0044] After the reaction is completed, the molar fraction of each component in the chlorination reactor is: 2,3-dichloro-1,1,1-trifluoropropane 0.996, 2,3,3-trichloro-1,1,1-trifluoro Propane 0.001, 3,3,3-trifluoropropene 0.003, use the material pump to transport the material in the photochlorination reactor to the rectification tower at a liquid phase flow rate of 50.8mol / h, and transfer the 3,3,3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com