Preparation method of chlorotoluene
A kind of chlorotoluene, chlorine technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
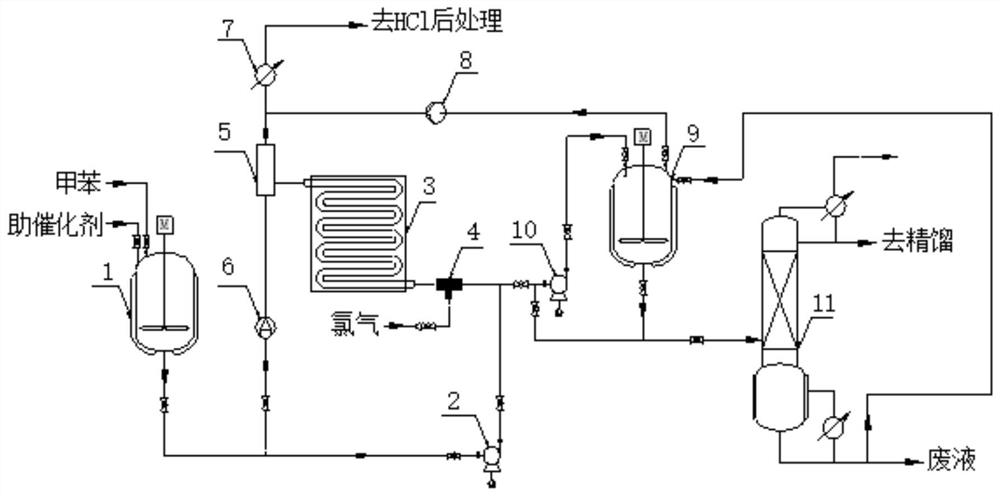
[0043] The plug flow reactor includes a circulating pump 2, a gas-liquid mixer 4, and an inner diameter of 32.0m long pipeline reactor 3 with jacket heat exchange, the inside of pipeline reactor 3 is filled with iron θ ring packing piled up porous iron packing bed; the preparation process of chlorotoluene is as follows:
[0044] 1) after toluene and cocatalyst 2-(phenylthio)thiophene are mixed according to mass ratio 1:0.0005, the mixture is added in pipeline reactor 3 using circulating pump 2, and the upper part of pipeline reactor 3 is continuously extracted using circulating pump 2 material, and into the bottom of the pipeline reactor 3, so that the mixture is forced to mix evenly;
[0045] 2) with the circulating liquid that the circulating pump 2 continuously extracts and the chlorine mass flow ratio be 1:0.055 feeding, and add the pipeline reactor 3 after mixing with the circulating liquid in the gas-liquid mixer 4, and the reaction temperature is 50 ℃. In the reaction...
Embodiment 2
[0049] The difference between this example and Example 1 is that in step 4), 75% to 95% of the high boiling point of the catalyst removal tower 11 tower kettle is recycled to the dechlorination and deacidification kettle in step (3) for reuse. The chlorine content is 0.05%, the high boiling total amount is 1.41%, the purity of ortho-chlorotoluene obtained by rectification is 99.87%, and the purity of p-chlorotoluene is 99.83%.
[0050] As can be seen from the above-mentioned example data, the high boiling amount generated after 75%~95% of the rectification raffinate is recycled and applied is reduced, the m-chlorotoluene content is reduced, and the product purity is improved. It can be seen from this that a certain amount of contained in the high boiling point of the catalyst-removing tower kettle is obtained. The amount of ferric chloride and co-catalyst can be applied to the dechlorination and deacidification kettle at high boiling point, which can further improve the reactio...
Embodiment 3
[0052] The difference between this example and Example 1 is that no catalyst is added to the reaction, and in step 3) sampling and analysis, it is found that the m-chlorine content is 3.13%, the total high-boiling amount is 7.58%, and the obtained o-chlorotoluene has a purity of 98.27%, p-chlorine The toluene purity was 97.35%.
[0053] Since no catalyst is added in this example, the final high boiling amount and the amount of m-chlorotoluene generated by hardening are high, and the product purity is reduced. It can be seen from this that the technical scheme of the present invention uses 2-(phenylthio)thiophene, 3-(phenylthiophene), 3-(phenyl) One or a mixture of thio) thiophene is a catalyst, which can reduce the generation of high boiling, the content of intermediate chlorotoluene in the reaction solution is lower than 0.10% after the reaction, and the purity of p-chlorotoluene obtained by rectification is greater than 99.7%;
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com