Synthesis method of 4-amino-2-hydroxy-3-isopropoxybenzoic acid
A technology of isopropoxybenzoic acid and synthesis method, applied in the direction of preparation of nitro compounds, chemical instruments and methods, preparation of carboxylate, etc., achieving the effects of high yield, simple operation and short route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
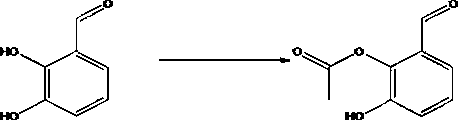
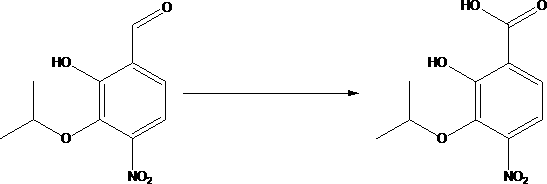
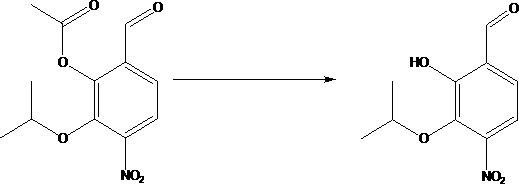
[0036] A kind of synthetic method of 4-amino-2-hydroxyl-3-isopropoxybenzoic acid, this synthetic method comprises the steps:
[0037] (1) Under low temperature conditions, use dichloromethane as a solvent, pour raw materials A and triethylamine into the solvent and mix evenly, add acetyl chloride dropwise, after the dropwise addition, heat up, stir and react to obtain compound B; low temperature is -18~-3℃, the mass ratio of compound A, acetyl chloride and triethylamine is: 1.5~2.5:1:2~4, the solid-liquid g / mL ratio of compound A and dichloromethane is 1:12~14 ;
[0038] (2) Add compound B to dimethylformamide, lower the temperature, add sodium hydrogen, heat up, react, cool, add isopropyl bromide dropwise, after the dropwise addition, heat up and react to obtain compound C; compound B, sodium hydrogen, The mass ratio of isopropyl bromide is 6~7:2.5~3:4~5, and the solid-liquid g / mL ratio of compound B and dimethylformamide is 1:10~15; the preparation of compound C solution is...
Embodiment
[0044] (1) Add 46g of compound A, 61g of triethylamine and 600mL of dichloromethane into the reaction flask, add 32g of acetyl chloride dropwise in an ice-water bath, after the drop is complete, heat up to 2,3°C and stir for 2h, detect by TLC, the reaction of the raw materials is complete , adding saturated sodium bicarbonate (300 ml), extracting with dichloromethane (500 ml*2), concentrating the organic phase, and column chromatography (mobile phase: n-hexane / ethyl acetate=50 / 1) to obtain 42.3 g of colorless Transparent oil, namely compound B, with a purity of 95.5% and a yield of 70.48%;
[0045] (2) Add 65g of compound B and dimethylformamide to the reaction bottle, cool to 0°C, add 30g of sodium hydrogen, react at 22°C for 2h, re-cool to 0°C, add 45g of isopropyl bromide dropwise, dropwise , heated to 25°C and reacted for 16 hours, TLC detection, the reaction of the raw materials was completed, the reaction solution was poured into 1000 ml of ice water, 6M hydrochloric aci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com