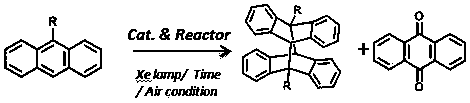
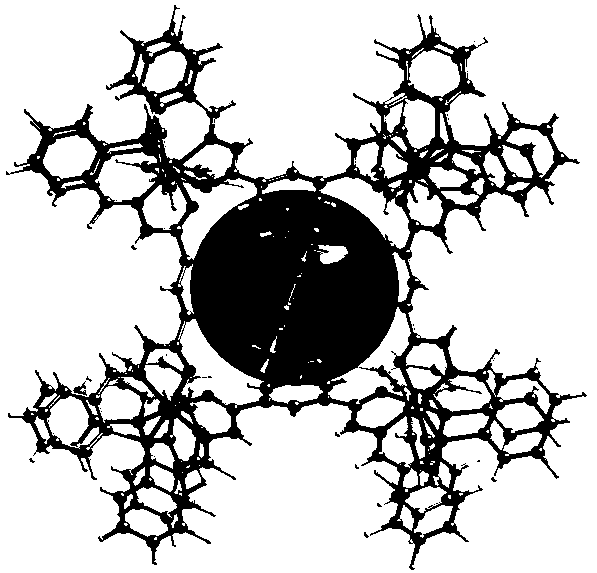
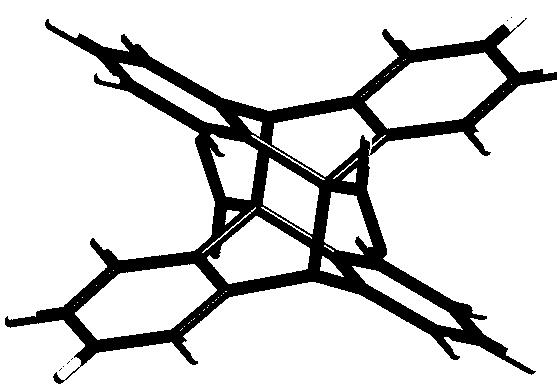
Method for preparing anthracycline dimer through photocatalysis
An anthracycline dimer, photocatalytic technology, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc. The effect of widening reaction conditions and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 9-anthracene formaldehyde (0.010 g, 0.05 mmol), cage compound Ni-L1 (1mol%) and tetrabutylammonium bromide TBABr (5 mol%) in a 25 mL test tube reactor, add mixed deuterium reagent CDCl 3 and DMSO- d 6 , the volume ratio is 1:6, under the atmosphere of air, after irradiating with 500 W xenon lamp for 8 hours, directly absorb the solution to detect the yield of its dimerization product by NMR, and analyze and detect the by-product by GC-MS, the final yield is 94 %, the selectivity is 92%.
Embodiment 2
[0030] Add 9-anthracenecarboxylic acid (0.011 g, 0.05 mmol), cage compound Ni-L1 (1mol%) and tetrabutylammonium bromide TBABr (5 mol%) in a 25 mL test tube reactor, add mixed deuterated reagent CDCl 3 and DMSO- d 6 , the volume ratio is 1:6, under the atmosphere of air, after irradiating with 500 W xenon lamp for 4 hours, directly absorb the solution to detect the yield of dimerization products by NMR, and use GC-MS to analyze and detect the by-products, the final yield is 96 %, the selectivity is 95%.
Embodiment 3
[0032] Add methyl 9-anthracenecarboxylate (0.012 g, 0.05 mmol), cage compound Ni-L1 (1 mol%) and tetrabutylammonium bromide TBABr (5 mol%) in a 25 mL test tube reactor, add mixed deuterium Reagent CDCl 3 and DMSO- d 6 , the volume ratio is 1:6, in the atmosphere of air, after irradiating with a 500 W xenon lamp for 8 hours, directly draw the solution for nuclear magnetic detection to detect the yield of dimerization products, and use GC-MS to analyze and detect by-products, the final yield is 100 %, the selectivity is 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com