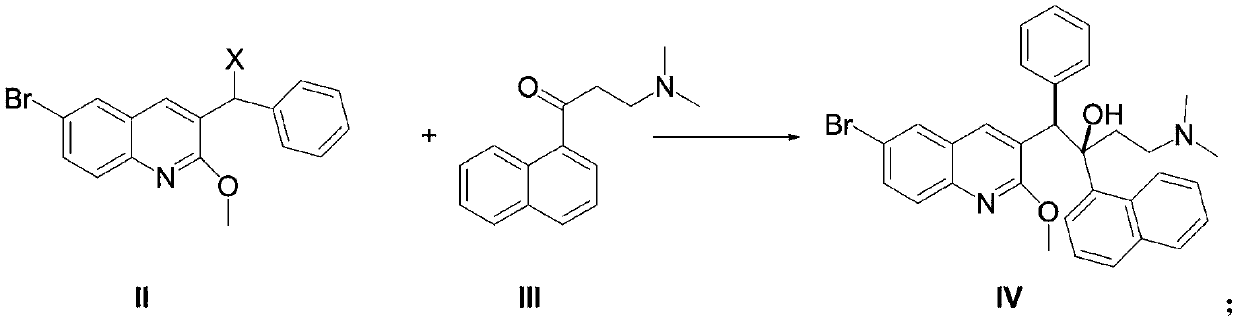
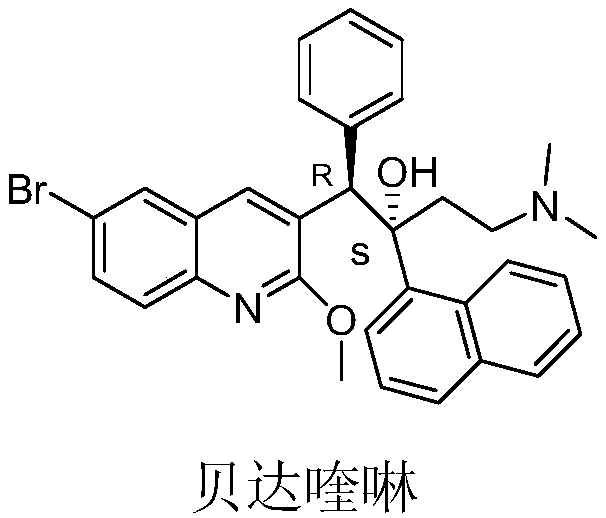
Preparation method of bedaquiline
A bedaquiline and compound technology, which is applied to the preparation field of bedaquiline, can solve the problems of long reaction steps, expensive reagents, harsh reaction conditions, etc., achieves simple operation, improves conversion rate and reaction yield, and reduces production cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1: Preparation of 6-bromo-3-chlorobenzyl-2-methoxyquinoline
[0053]
[0054]Dissolve 3-benzyl-6-bromo-2-methoxyquinoline (32.7g, 0.01mol, 1eq) in 200ml of 1,2-dichloroethane, add NCS (14.7g, 1.1eq), peroxide Benzoyl (1.5 g, 0.006 mol), replaced with argon. The reaction system was heated to 80° C., and the reaction was continued at this temperature for 5 hours. After the reaction was complete, cool, add water and extract with dichloromethane, anhydrous Na 2 SO 4 After drying, the organic phase was concentrated, and the residue was recrystallized from methanol to obtain compound II-1 (29.3 g, yield 81%). 1 H NMR (400MHz, CDCl 3 )δ7.87(s,1H),7.84(d,J=4.0Hz,1H),7.63-7.70(m,2H),7.29–7.41(m,5H),6.05(s,1H),4.04(s ,3H). Ms(+C,ESI): M=362, found value: 363(M+1).
Embodiment 2
[0055] Embodiment 2: Preparation of 6-bromo-3-bromobenzyl-2-methoxyquinoline
[0056]
[0057] Dissolve 3-benzyl-6-bromo-2-methoxyquinoline (32.7g, 1eq) in 200ml of dichloromethane, add NBS (18.7g, 1.05eq), benzoyl peroxide (1.5g) , argon replacement. It was heated to reflux, and the reaction was continued at this temperature for 5 hours. After the reaction was complete, cool, add water and extract with dichloromethane, anhydrous Na 2 SO 4 After drying, the organic phase was concentrated, and the residue was recrystallized from methanol to obtain compound II-2 (34.6 g, yield 85%). 1 H NMR (400MHz, CDCl 3 )δ8.10(s,1H), 7.87(d,J=4.0Hz,1H), 7.44–7.69(m,4H), 7.26–7.38(m,3H), 6.56(s,1H),4.06(s , 3H); Ms(+C, ESI): M=406, found: (407, M+1).
Embodiment 3
[0058] Embodiment 3: the preparation of bedaquiline (racemate)
[0059]
[0060] Zinc powder (6.5g, 2eq) was suspended in 20ml of anhydrous tetrahydrofuran, trimethylchlorosilane (0.2ml) was slowly added dropwise at room temperature, and stirred for 5min after the addition was complete. Then the temperature was raised to 60°C, and at this temperature, 90ml of anhydrous THF mixed solution prepared by compound II-1 (18.2g, 1eq) and compound III (17g, 1.5eq) was slowly added dropwise, and the addition was completed within 1h. Stirring was continued for 4h after addition. Stop heating, cool to room temperature, extract with ethyl acetate, anhydrous Na 2 SO 4 Dry, stop the concentration when the organic phase is concentrated to 50ml, put it in an ice-water bath and stir, and precipitate the diastereomer B, that is, (S, S)-bedaquiline and (R,R)-bedaquiline non-corresponding The mixture of isomers was suction filtered, washed with a small amount of ethyl acetate, the solid was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com