Preparation method of dammarane saponin and application of dammarane saponin in preparation of hypoglycemic and anti-inflammatory drugs and health products
A dammarane type, anti-inflammatory drug technology, applied in the field of biomedicine, to achieve the effect of good hypoglycemic and anti-inflammatory activities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] A method for preparing dammarane-type saponins, comprising the following steps:
[0100] S1: Take 10 kg of dried Cyclocarya paliurus leaves, add 75% ethanol aqueous solution, heat and reflux for extraction 3 times in sequence for 3 hours, 2 hours, and 2 hours, combine the filtrates, recover ethanol under reduced pressure, and obtain 2.10 kg of extract;
[0101] S2: Suspend 1.5kg of extract in 20L of water, absorb on D101 macroporous adsorption resin chromatographic column, wash with water, and then elute with 30% and 95% ethanol to obtain 250g and 625.0 g of eluate of 30% and 95% ethanol respectively g, The eluate is taken as the Cyclocarya paliurus leaf extract rich in dammarane-type triterpene saponins.
Embodiment 2
[0103] Isolation, preparation and structure identification of dammarane-type triterpene saponins 1-11.
[0104] Compounds 1-11 were isolated simultaneously and thus described in one example.
[0105] [Isolation and preparation of compound 1-11]
[0106] Get 95% ethanol eluate 150.0g obtained in the above-mentioned Example 1, through silica gel column chromatography, dichloromethane-methanol (100:0, 60:1, 20:1, 10:1, 8:1 , 4:1, 2:1, 0:100) as the eluent for gradient elution to obtain Fr.A (22g), Fr.B (16g), Fr.C (23g), Fr.D (15g) , Fr.E (27g), Fr.F (19g), Fr.G (13g) and Fr.H (6g). Component Fr.F (19g) was subjected to reverse-phase C18 silica gel column chromatography, using water-methanol (100:0-0:100) as the eluent for gradient elution, and 6 components were obtained as Fr.F. 1(0.5g), Fr.F.2(3.5g), Fr.F.3(4.2g), Fr.F.4(2.5g), Fr.F.5(1.5g) and Fr.F. 6 (1.1 g). Component Fr.F.3 (4.2g) was subjected to reverse phase C18 silica gel column chromatography (MeOH-H2O, 60:40→100:...
Embodiment 3
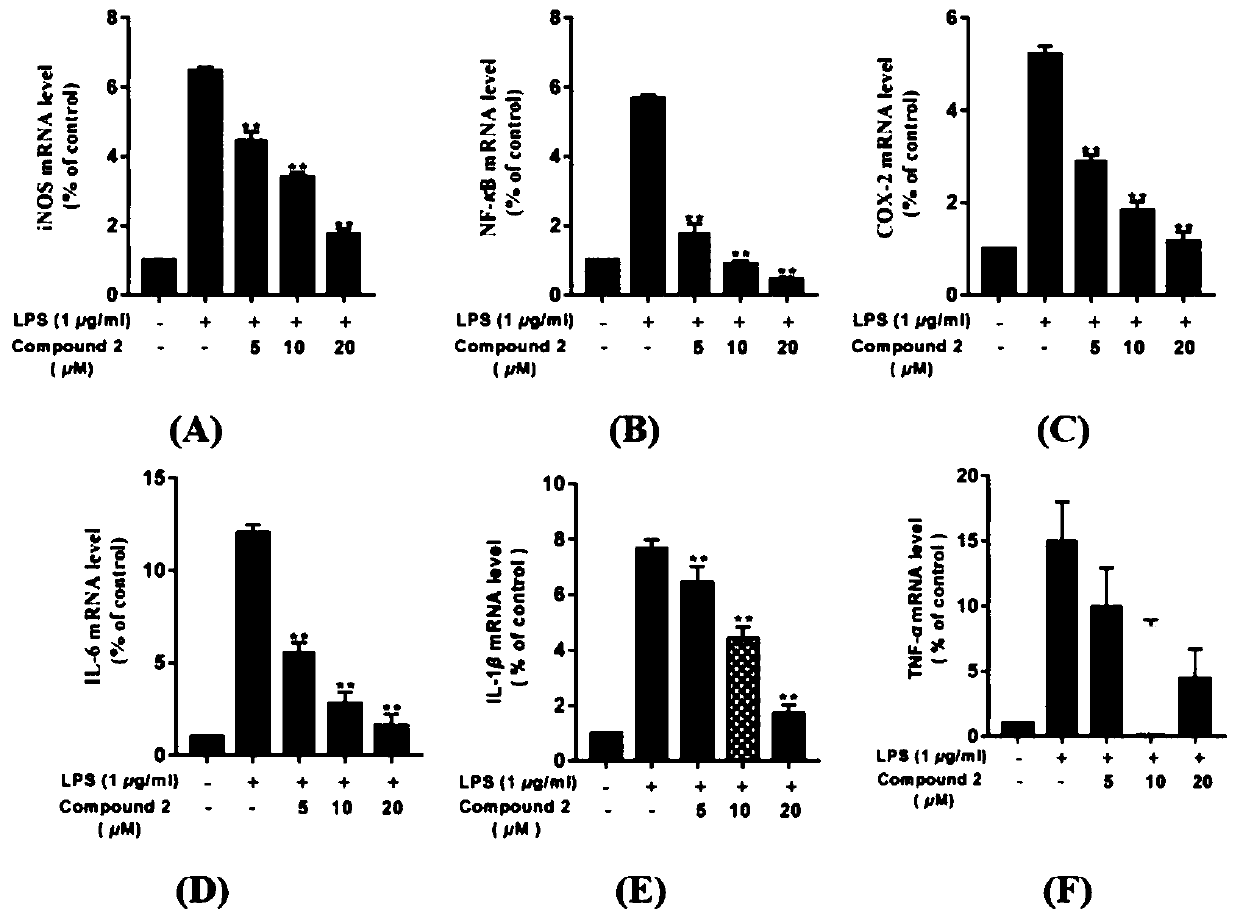
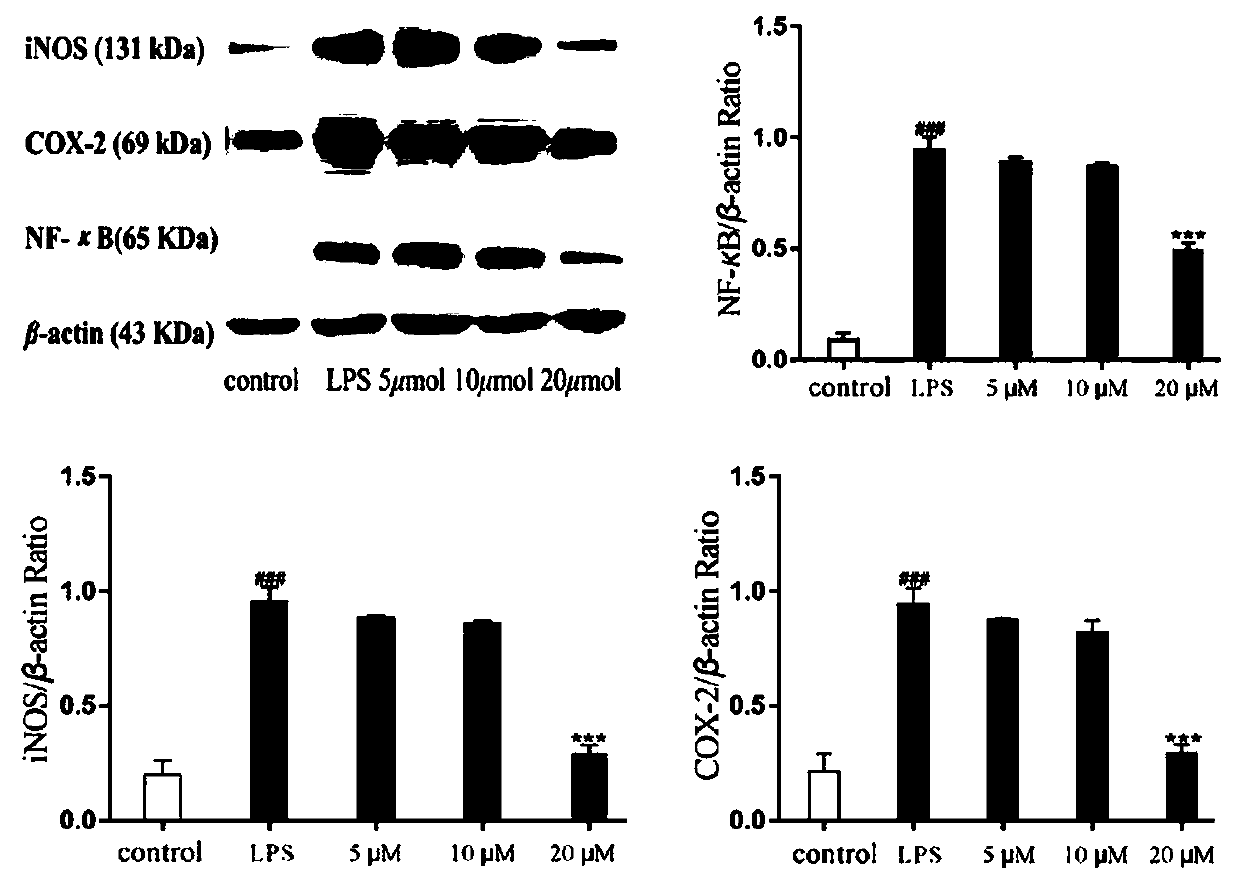
[0133]Determination of biological activity of dammarane-type triterpene saponins 1-11
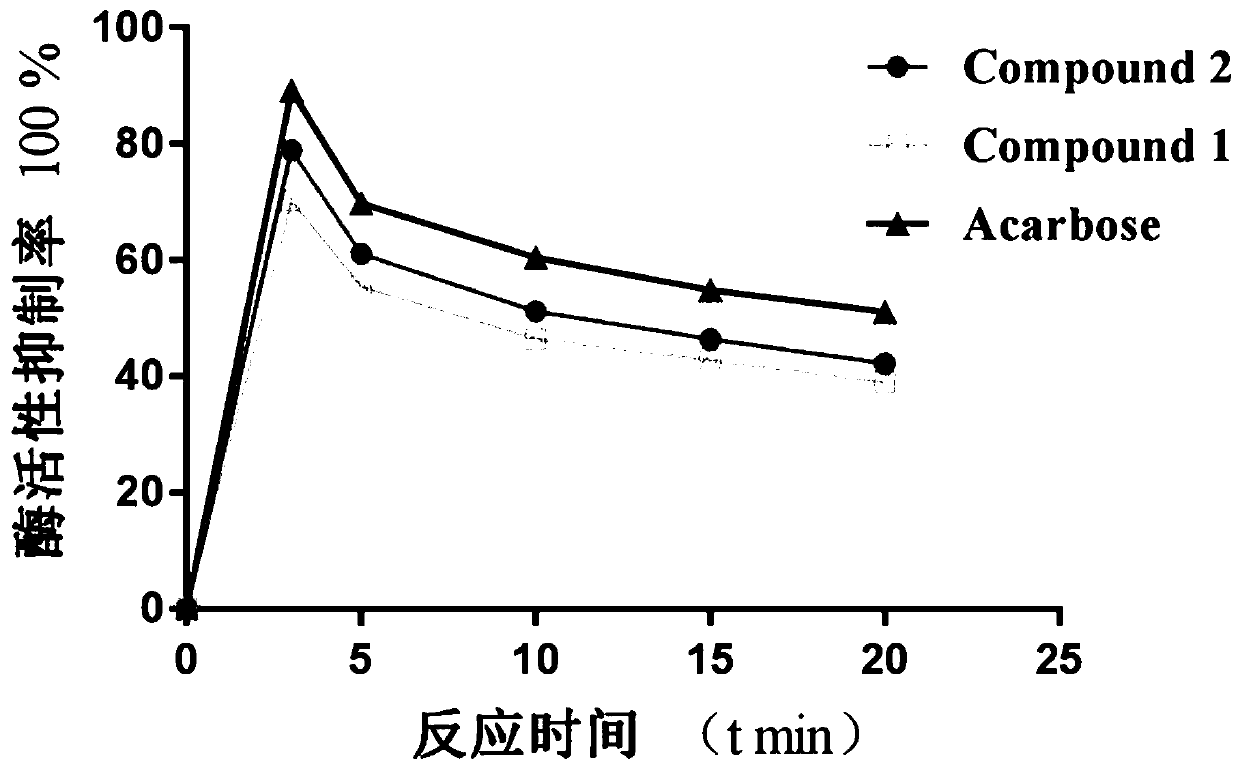
[0134] 3.1 Compound 1-11 inhibits α-glucosidase activity and the determination of compound 1 and 2 on enzyme activity and reaction time
[0135] 3.1.1 Determination of inhibition rate of α-glucosidase activity
[0136] In the test, acarbose was used as the positive control drug, and all the compounds to be tested were diluted with phosphate buffer (pH=6.9) to different concentrations (0.001, 0.01, 0.05, 0.1, 0.2, 0.5 mg·mL -1 ) sample solution. In a 96-well plate, add 50 μL of compound solutions of various concentrations, 50 μL of phosphate buffer, and then add 100 μL of 0.2 U·mL -1 α-glucosidase solution, incubated at 37°C for 10min, then added 50μL 2mmol·L -1 The p-nitrophenyl-β-D-galactopyranoside (PNPG) was further incubated at 37°C for 5 minutes, and finally the absorbance (A) value was measured at a wavelength of 405nm. Inhibition rate (%) = [A 1 -(A 2 -A 3 )] / A 1 *100%, where...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com