Bifunctional catalyst, preparation and application of bifunctional catalyst in one-step synthesis of 2, 5-furan dialkyl ether from 5-hydroxymethylfurfural
A bifunctional catalyst and compound technology, applied in the field of molecular sieves, can solve problems such as complex operation and BHMF loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
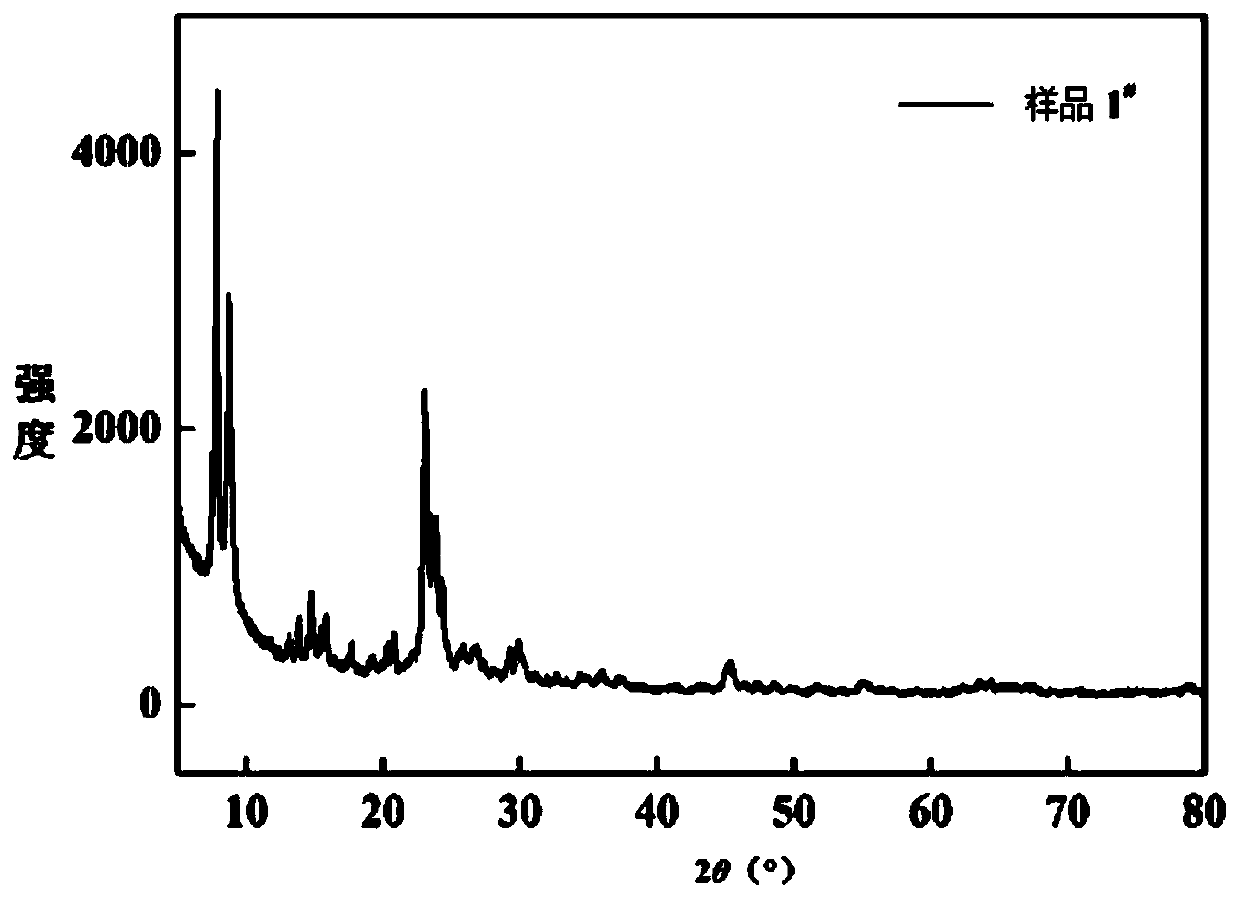
[0097] Example 1 Sample 1 # preparation
[0098] Mix 0.24g of aluminum isopropoxide, 12g of tetrapropylammonium hydroxide, 13mL of ethyl orthosilicate, 1.6mL of octadecyltrimethoxysilane and 50mL of ethanol in a beaker, and stir at room temperature until a gel is formed; The gel was dried at room temperature for 160 hours, and the dried gel was transferred to a 50ml liner, and then transferred to a 250mL stainless steel hydrothermal kettle containing 50ml deionized water, and crystallized at 180°C for 80h under a water vapor atmosphere; after filtration and washing , after drying at 110°C for 3 hours and calcining at 550°C for 8 hours, the hierarchically porous ZSM-5 molecular sieve sample was obtained, which was designated as sample 1 # .
Embodiment 2
[0099] Example 2 Sample 2 # preparation of
[0100] Mix 0.24g of aluminum isopropoxide, 12g of tetrapropylammonium hydroxide, 13mL of ethyl orthosilicate, 1.6mL of hexadecyltrimethoxysilane and 50mL of ethanol in a beaker, and stir at room temperature until a gel is formed; The gel was dried at room temperature for 160 hours, and the dried gel was transferred to a 50ml inner liner, and then transferred to a 250mL stainless steel hydrothermal kettle containing 50ml deionized water, and crystallized at 180°C for 70 hours under a water vapor atmosphere; after filtration and washing After drying at 110° C. for 4 hours and calcination at 550° C. for 7 hours, the hierarchically porous ZSM-5 molecular sieve sample was obtained. Take 0.8g copper nitrate trihydrate and add it to 4mL deionized water, then add 4g hierarchical porous ZSM-5 molecular sieve 1 # , impregnated for 48 hours, dried at 80°C for 2 hours, calcined at 480°C for 6 hours, and reduced at 400°C for 6 hours in a hydro...
Embodiment 3
[0101] Example 3 Sample 3 # preparation of
[0102] Mix 0.06g of aluminum isopropoxide, 12g of tetrapropylammonium hydroxide, 13mL of ethyl orthosilicate, 1.6mL of hexadecyltrimethoxysilane and 50mL of ethanol in a beaker, and stir at 25°C until a gel is formed; The gel was dried at room temperature for 160 hours, and the dry gel was transferred to a 50ml liner, and then transferred to a 250mL stainless steel hydrothermal kettle containing 50ml deionized water, and crystallized at 170°C for 90h under a water vapor atmosphere; filtered and washed After drying at 110°C for 2 hours and calcination at 550°C for 7 hours, the hierarchically porous ZSM-5 molecular sieve sample was obtained, which was designated as sample 3 # .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com