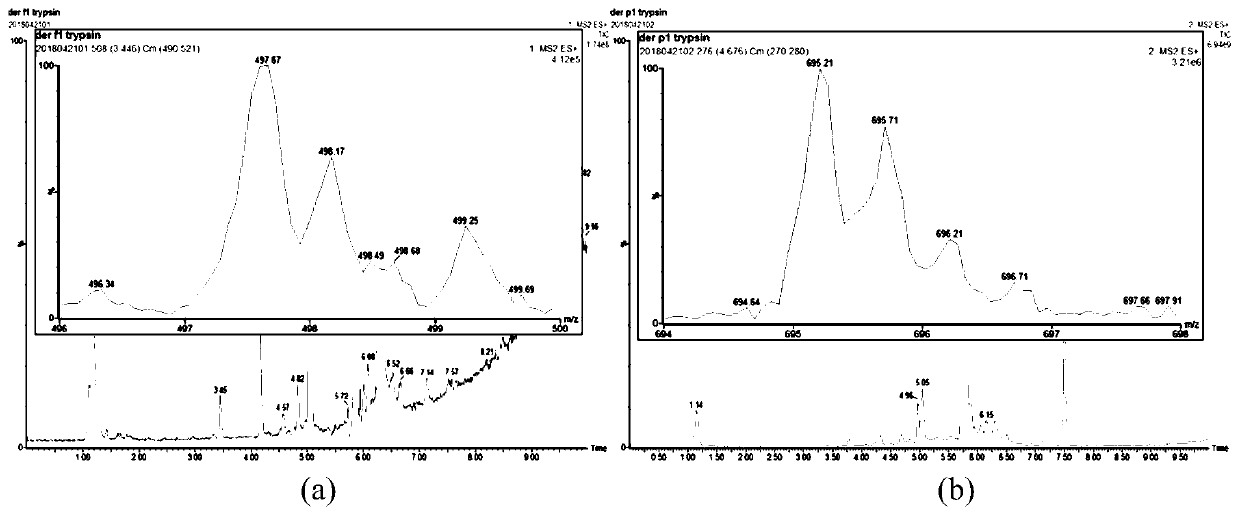
Method for detecting dust mite allergen proteins Der f1 and Der p1 and nitrification products thereof in dust
A technology of allergen protein and detection method, which is applied in the field of detection of dust mite allergen proteins Der f1 and Der p1 and their nitration products in dust, to achieve the effect of improving accuracy and flux and ensuring accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 sample analysis process
[0027] 1.1 Selection of characteristic polypeptides of dust mite allergen protein
[0028] Take 10 μg dust mite allergen standard protein (Der f1 and Der p1) solution in a 1.5mL low-protein adsorption tube, add 20 μL acetonitrile, 20 μL 50 mmol·L -1 NH 4 HCO 3 Buffer solution, placed at 37°C for 15 min; add 5 μL reducing solution (45 mmol L -1 DTT and 20mmol·L -1 TCEP dissolved in 50mmol·L -1 NH 4 HCO 3 buffer), placed at 37°C for 30min; add 5μL of 100mmol·L -1 IAA solution, mix and place for 30min at 37°C in the dark; add 5.5 μL 90mmol L -1 DTT stock solution was used to quench the residual IAA; add 134.5 μL of ultrapure water; add 5 μL of trypsin (20 ng·μL -1 , diluted in HCl) so that the enzyme / substrate ratio was 1:100 (w / w). After incubating at 37°C for 24 hours, add 2 μL of 10% formic acid / water (v / v), vortex and mix well, and place at room temperature for 10 minutes; ) solution to redissolve. Then 14000r·min at 4°C ...
Embodiment 2
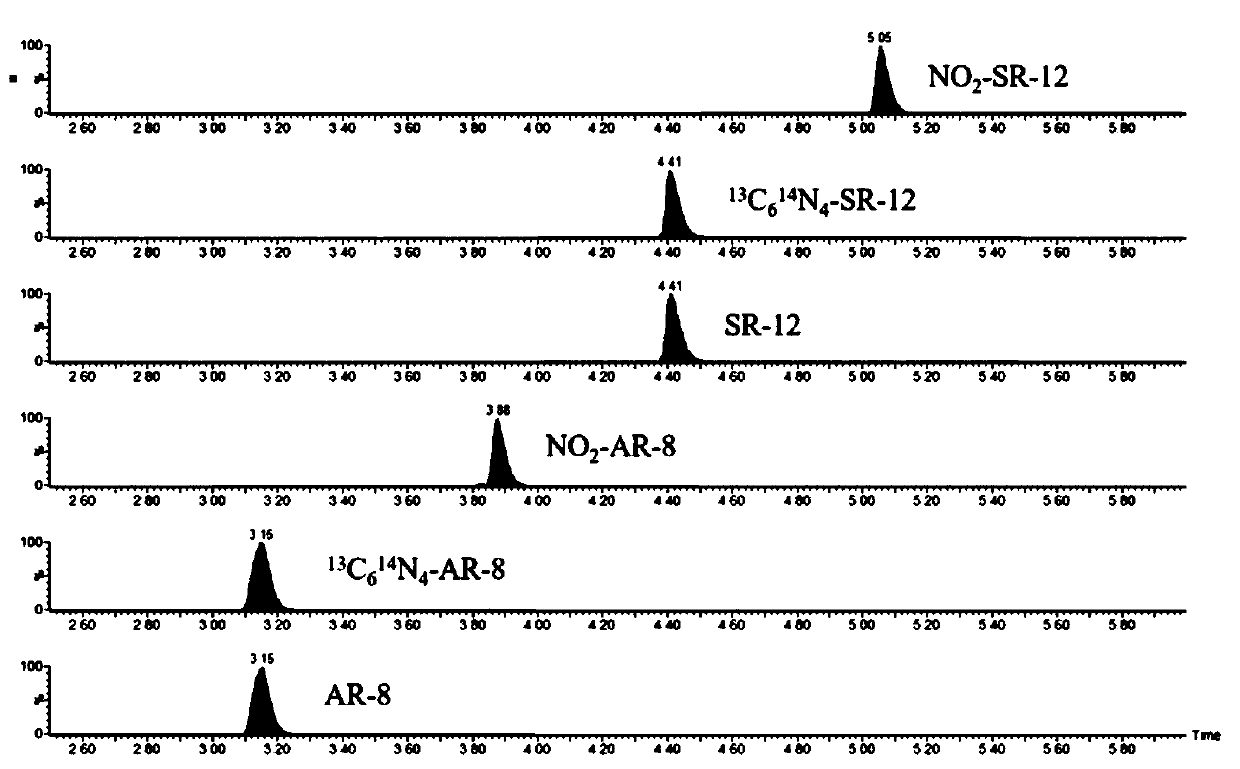
[0054] Embodiment 2 method reliability evaluation
[0055] 2.1 Solution preparation
[0056] The peptide mixed standard solution configuration is as follows:
[0057] (1) Use 11% acetonitrile / water (v / v) solution to prepare a concentration of 1mg·mL -1 AR-8 stock solution, dilute the single standard stepwise to 100μg·mL -1 , 10μg·mL -1 , 1 μg·mL -1 ; Prepare 1 mg·mL with 15% acetonitrile / water (v / v) solution -1 The SR-12 mother liquor and NO 2 - SR-12 stock solution, dilute the single standard stepwise to 100 μg mL -1 , 10μg·mL -1 , 1 μg·mL -1 ; Use 25% acetonitrile / water (v / v) solution to prepare a concentration of 1 mg·mL -1 NO 2 -AR-8 stock solution, dilute the single standard stepwise to 100 μg·mL -1 , 10μg·mL -1 , 1 μg·mL -1 ;
[0058] (2) High-concentration peptide mixed standard solution: take 100 μL of 1 μg·mL respectively -1 AR-8, SR-12, NO 2 -AR-8 and NO 2 - SR-12 The four peptides are single-labeled in a low-protein adsorption tube and diluted with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com