Preparation method of lithium tetrafluoroborate phosphate, non-aqueous electrolyte and battery
A technology of lithium tetrafluorooxalate phosphate and non-aqueous electrolyte, applied in electrolytic capacitors, secondary batteries, chemical instruments and methods, etc., can solve the problems of high chlorine compounds, difficult separation, complicated reactions, etc., and achieves low cost and high price. Inexpensive, easy-to-use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
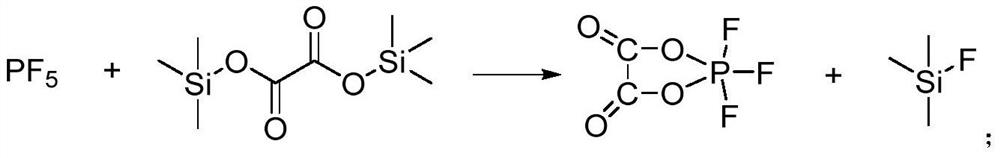
[0061] In a glove box, take 30 g (0.128 mol) of bis(trimethylsilyl) oxalate with a moisture content of less than 50 ppm in a three-necked flask, add 130 mL of acetonitrile with a moisture content of less than 10 ppm dried with molecular sieves, and dissolve to obtain bis(trimethylsilyl) oxalate. (trimethylsilyl)oxalate solution.
[0062] The solution was removed from the glove box, and 0.134 mol of phosphorus pentafluoride gas was introduced at 60° C. (the molar ratio of bis(trimethylsilyl) oxalate to phosphorus pentafluoride was 1:1.05) to participate in the reaction. After the addition was completed, stirring was continued for 2 h and the reaction was completed to obtain the first reaction mixture.
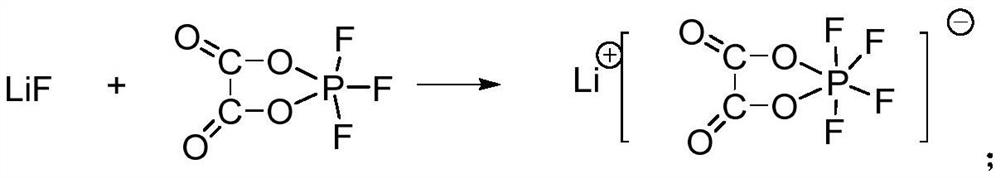
[0063] 3.64 g (0.14 mol) of dry lithium fluoride was added to the first reaction mixture, and then in a nitrogen atmosphere, the temperature was raised to 90° C. to carry out the reaction for 6 h. After the reaction was completed, the second reaction mixture was obtained.
[00...
Embodiment 2
[0070] In the glove box, take 60 g (0.256 mol) of bis(trimethylsilyl) oxalate with a moisture content of less than 50 ppm in a three-necked flask, add 430 mL of dimethyl carbonate with a moisture content of less than 10 ppm dried with molecular sieves, Dissolution gave a solution of bis(trimethylsilyl)oxalate.
[0071] The solution was removed from the glove box, and 0.28 mol of phosphorus pentafluoride gas was introduced at 80°C (the molar ratio of bis(trimethylsilyl) oxalate to phosphorus pentafluoride was 1:1.1) to participate in the reaction. After the addition was completed, stirring was continued for 10 h and the reaction was completed to obtain the first reaction mixture.
[0072] 7.54 g (0.29 mol) of dry lithium fluoride was added to the first reaction mixture, and then in a nitrogen atmosphere, the temperature was raised to 85° C. for 8 hours, and the second reaction mixture was obtained after the reaction was completed.
[0073] The second reaction mixture was subje...
Embodiment 3
[0079] In the glove box, take 100 g (0.427 mol) of bis(trimethylsilyl) oxalate with a moisture content of less than 50 ppm in a three-necked flask, add 540 mL of dimethyl carbonate with a moisture content of less than 10 ppm dried with molecular sieves, Dissolution gave a solution of bis(trimethylsilyl)oxalate.
[0080] The solution was removed from the glove box, and 0.49 mol of phosphorus pentafluoride gas was introduced at 85° C. (the molar ratio of bis(trimethylsilyl) oxalate to phosphorus pentafluoride was 1:1.16) to participate in the reaction. After the addition was completed, stirring was continued for 6 h and the reaction was completed to obtain the first reaction mixture.
[0081] 16.6 g (0.64 mol) of dry lithium fluoride was added to the first reaction mixture, and then in a nitrogen atmosphere, the temperature was raised to 90° C. for 8 h, and the second reaction mixture was obtained after the reaction was completed.
[0082] The second reaction mixture was subjec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com