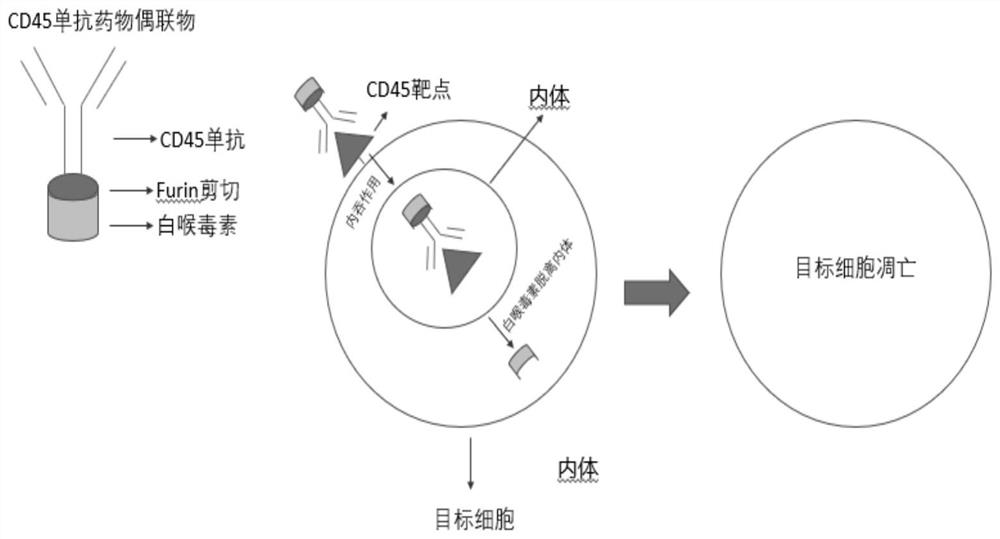
Antibody-drug conjugate of targeted CD45 and preparation and application thereof
An antibody drug and conjugate technology, applied in the biological field, can solve problems such as affecting the cure rate of blood system diseases, and achieve the effects of low side effects, increased scope, and reduced burden on patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
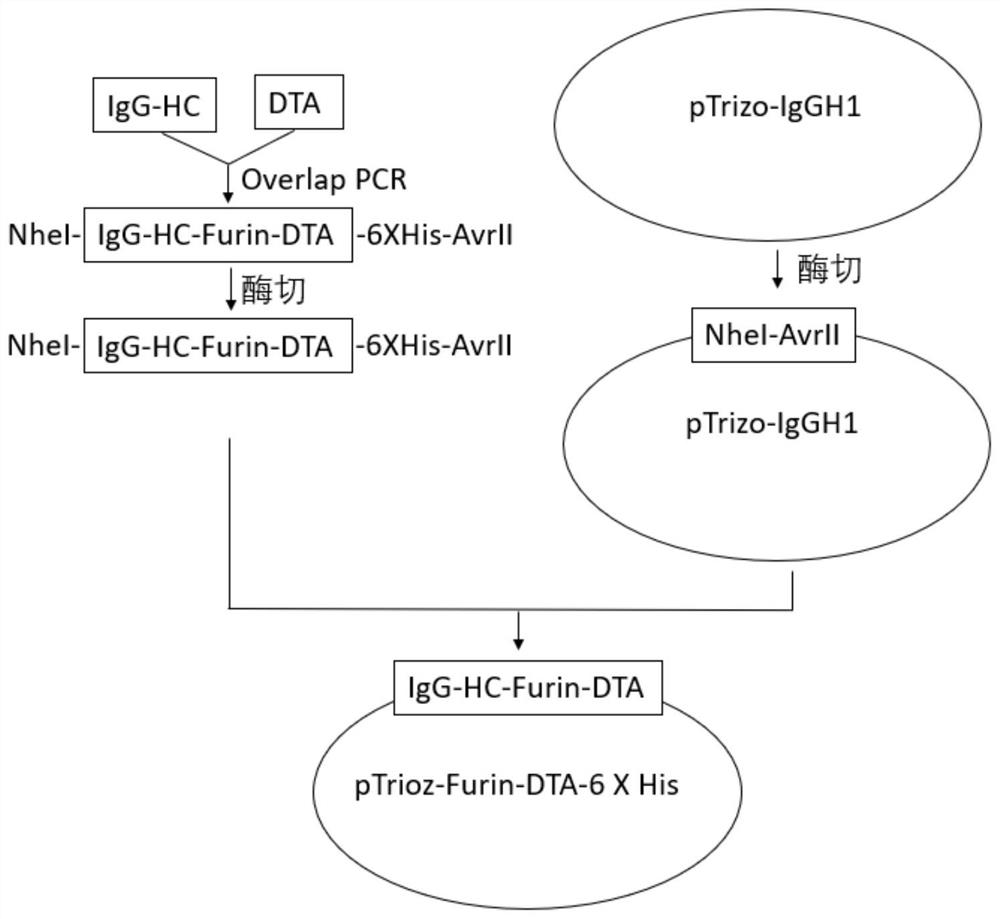
[0057] Construct the production vector of pTrioz-Furin-DTA-6 X His, the overall process is as attached image 3 shown.
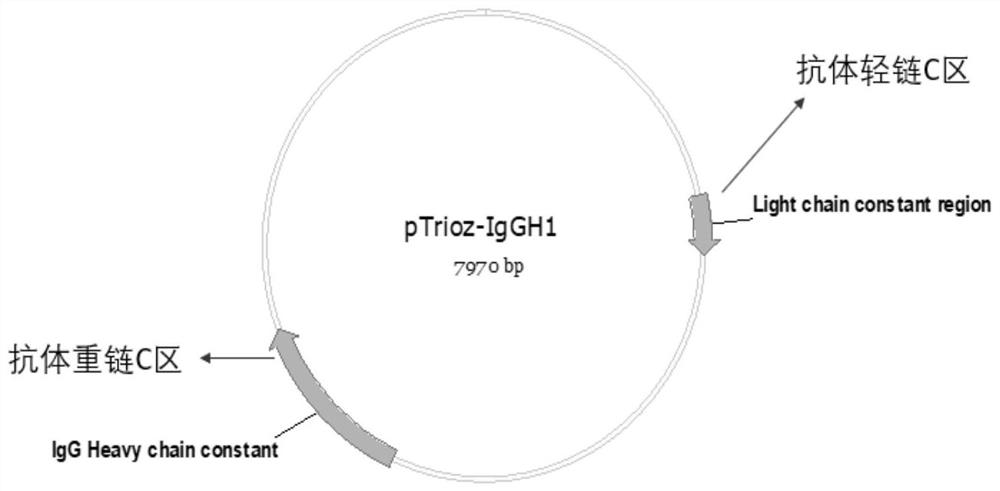
[0058] (1) Use the pTrioz blank plasmid as a template, as attached figure 2 As shown, it contains the C region of human IgG1 light chain and the C region of human IgG1 heavy chain, using the forward primer whose sequence is shown in SEQ ID NO.4
[0059] IgHC-F-NheI- GCTAGC ACCAAGGGCCCATCGGTCTTCCCCC and the reverse primer whose sequence is shown in SEQ ID NO.5
[0060] IgHC-R-Furin-TGACAATGAGCTACCTACTGATCGCCTGACACGATTTCCTGCTTTACCCGGAGACAGGGAGAGGC was used for PCR to amplify the gene fragment IgHC with the sequence shown in SEQ ID NO.6; the underlined sequence is the restriction site.
[0061] (2) Using the pIRES-proHB EGF WT plasmid (addgene) as a template, use the forward primer whose sequence is shown in SEQ ID NO.7
[0062] DTA-F-Furin-GCAGGAAATCGTGTCAGGCGATCAGTAGGTAGCTCATTGTCAGGCGCTGATGATGTTGTTGATTC and the reverse primer DTA-R-6XHis-AvrII-CCTAGGGTG...
Embodiment 2
[0075] A commercial company synthesizes the CD45 Ab light chain with the sequence shown in SEQ ID NO.11;
[0076] Using the synthetic product as a template, using a forward primer whose sequence is shown in SEQ ID NO.12CD45 Ab-L-F-NcoI-CCATGGCAATGATGTCCTCTGCTCAG and a reverse primer whose sequence is shown in SEQ ID NO.13 CD45 Ab-L-R-BsiWI-CGTACGTTTGATTTCCAGCTTGGTGC PCR, amplifying the gene fragment CD45 Ab L whose sequence is shown in SEQ ID NO.14;
[0077] The PCR product was cloned into the vector pTrioz-Furin-DTA-6 X His using restriction sites NcoI and BsiWI to obtain the intermediate vector pTrioz-L-Furin-DTA-6 X His, as shown in SEQ ID NO.15, the construction process as attached Figure 5 shown.
[0078] The PCR reaction system is as follows:
[0079]
[0080] The PCR amplification conditions are:
[0081]
[0082] The target gene fragment CD45 L was cloned into the vector pTrioz-Furin-DTA-6 X His: After the PCR products were separated by agarose gel electroph...
Embodiment 3
[0084] Insertion of CD45 Ab heavy chain includes the following steps:
[0085] A commercial company synthesizes the CD45 Ab heavy chain with the sequence shown in SEQ ID NO.16;
[0086]Using the synthetic product as a template, using the forward primer GATATCATGAGCGTGCTGATTCTTTTGTGGC whose sequence is shown in SEQ ID NO.17 CD45 Ab-H-F-EcoRV and the reverse primer whose sequence is shown as SEQ ID NO.18 CD45 Ab-H-R-NheI-GCTAGCTACTGGTACTTCGATGTCTG PCR, amplifying the gene fragment CD45 Ab H whose sequence is shown in SEQ ID NO.19;
[0087] The PCR reaction system is as follows:
[0088]
[0089] The PCR amplification conditions are:
[0090]
[0091] The target gene fragment CD45 H was cloned into the vector pTrioz-L-Furin-DTA-6 X His: After the PCR products were separated by agarose gel electrophoresis, the target bands were excised and recovered using AxyPrep DNA Gel Extraction Kit (Axygen). Purified CD45 Ab H and carrier pTrioz-L-Furin-DTA-6 X His were digested with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com