Preparation method of high-entropy oxide material
An oxide and high-entropy technology, which is applied in the field of preparation of high-entropy oxide materials, can solve the problem that high-entropy oxide materials have not yet been retrieved, and achieve the effects of uniform morphology, mild reaction conditions and reduced energy consumption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
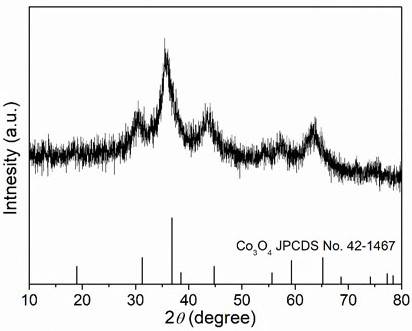
[0022] Weigh 2.424 g of Fe(NO 3 ) 3 9H 2 O, 2.25 g of Al(NO 3 ) 3 9H 2 O, 1.746 g of Co(NO 3 ) 2 ·6H 2 O, 1.746 g of Ni(NO 3 ) 2 ·6H 2 O, 1.782 g of Zn(NO 3 ) 2 ·6H 2 O was dissolved in 60 mL deionized water and stirred evenly to obtain a mixed solution of metal salts; then 0.72 g of urea was weighed and added to the above mixed solution, and stirred evenly; then the above mixed solution was transferred to a polytetrafluoroethylene-lined reactor , heated to 120°C, reacted for 1 h, cooled to room temperature, filtered the reaction solution with deionized water, and washed three times with deionized water to obtain a hydroxide solid powder; finally put the above solid powder into a low-temperature plasma reactor for reaction 20 min (the reaction atmosphere is oxygen, the radio frequency power is 300 W), and the high entropy oxide is obtained. XRD spectrum ( figure 1 ) shows that the prepared (FeAlCoNiZn) 3 o 4 High-entropy oxides have a spinel structure.
Embodiment 2
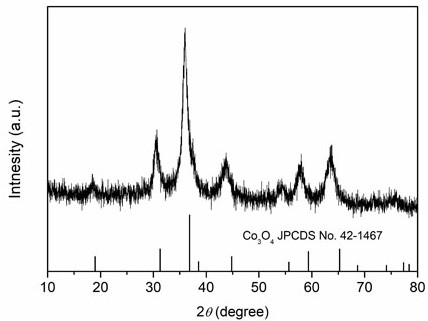
[0024] Weigh 2.424 g of Fe(NO 3 ) 3 9H 2 O, 2.4 g of Cr(NO 3 ) 3 9H 2 O, 1.746 g of Co(NO 3 ) 2 ·6H 2 O, 1.746 g of Ni(NO 3 ) 2 ·6H 2 O, 1.782 g of Zn(NO 3 ) 2 ·6H 2 O was dissolved in 60 mL deionized water and stirred evenly to obtain a mixed solution of metal salts; then 2.52 g of hexamethylenetetramine was weighed and added to the mixed solution, and stirred evenly; then the mixed solution was transferred to polytetrafluoroethylene In an ethylene-lined reactor, heat to 140°C, react for 1 h, and after cooling to room temperature, filter the reaction solution with suction and wash with deionized water three times to obtain a hydroxide solid powder; finally put the above solid powder into a low-temperature plasma The high-entropy oxide was obtained by reacting in a bulk reactor for 20 min (the reaction atmosphere was oxygen, and the radio frequency power was 400 W). XRD spectrum ( figure 2 ) shows that the prepared (FeCrCoNiZn) 3 o 4 High-entropy oxides have ...
Embodiment 3
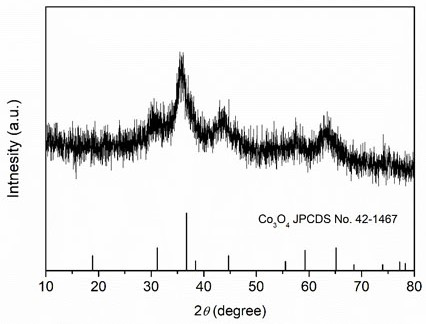
[0026] Weigh 2.424 g of Fe(NO 3 ) 3 9H 2 O, 2.4 g of Cr(NO 3 ) 3 9H 2 O, 1.746 g of Co(NO 3 ) 2 ·6H 2 O, 1.746 g of Ni(NO 3 ) 2 ·6H 2 O, 1.45 g of Cu(NO 3 ) 2 ·6H 2 O was dissolved in 60 mL deionized water and stirred evenly to obtain a mixed solution of metal salts; then 2.09 g of arginine was weighed and added to the mixed solution of metal salts, and stirred evenly; then the mixed solution was transferred to a polytetrafluoroethylene liner In the reaction kettle, heat to 100°C, react for 1 h, and after cooling to room temperature, filter the reaction solution with suction and wash with deionized water for 3 times to obtain a hydroxide solid powder; finally put the above solid powder into a low-temperature plasma reactor The high-entropy oxides were obtained after 20 min reaction (the reaction atmosphere was oxygen, and the radio frequency power was 400 W). XRD spectrum ( image 3 ) shows that the prepared (FeCrCoNiCu) 3 o 4 High-entropy oxides have a spinel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com