Preparation method of hierarchical porous metal-organic framework material for drug-loaded sustained release
A metal-organic framework, multi-level pore technology, applied in pharmaceutical formulations, medical preparations with inactive ingredients, etc., can solve the problems of decreased stability of MOFs, unsuitable for biomaterial preparation, interlaced framework networks, etc., to promote nucleation. Effect of crystallization process, structural stability, high specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1. Preparation of Metal Organic Framework (HP-Cu-BTC for short)
[0026] The preparation method of the metal-organic framework material for drug-loaded sustained release of the present invention comprises the following steps:
[0027] (1) Take 0.5mL TX-100 and 3mL 1-butyl-3-methylimidazolium hexafluoroborate successively into the reaction flask, mix and stir for 10min, then add 9mL propylene glycol, stir for 30min to obtain an ionic liquid microemulsion.
[0028] (2) 188mg metal salt anhydrous copper nitrate [Cu(NO 3 ) 2 ], 104 mg of organic ligand trimesic acid were poured into the ionic liquid microemulsion in turn, stirred for 1 to 2 min, then transferred to a high-pressure reactor, and fed with CO at a pressure of 3 MPa 2 , reacted for 3 hours, after the reaction, washed with ethanol for 3 times, and then dried in an oven at 60° C., and HP-Cu-BTC was obtained after drying.
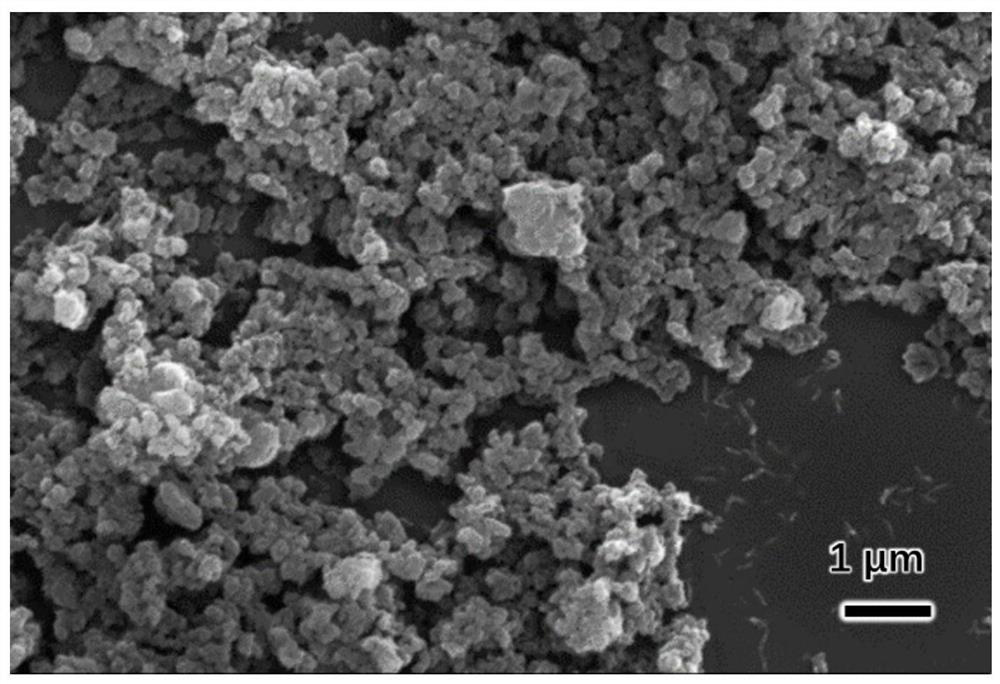
[0029] The SEM figure of metal organic material (HP-Cu-BTC) of the present invention is a...
Embodiment 2
[0036] 1. Preparation of HP-Cu-BTC
[0037] The preparation method of the hierarchically porous metal-organic framework material for drug-loaded sustained release of the present invention comprises the following steps:
[0038] (1) Take 1mL TX-100 and 4mL 1-butyl-3-methylimidazolium tetrafluorophosphate successively into the reaction flask, mix and stir for 10min, then add 8mL ethanol, and stir for 30min.
[0039] (2) 250mg of anhydrous copper sulfate [Cu(SO 4 ) 2], 208mg of trimesic acid were poured into the ionic liquid microemulsion in turn, stirred for 1-2min, transferred to a high-pressure reactor, and 5MPa CO 2 , Reaction 2h. After the reaction, it was washed with ethanol for three times, and dried in an oven at 60° C. to obtain HP-Cu-BTC after drying.
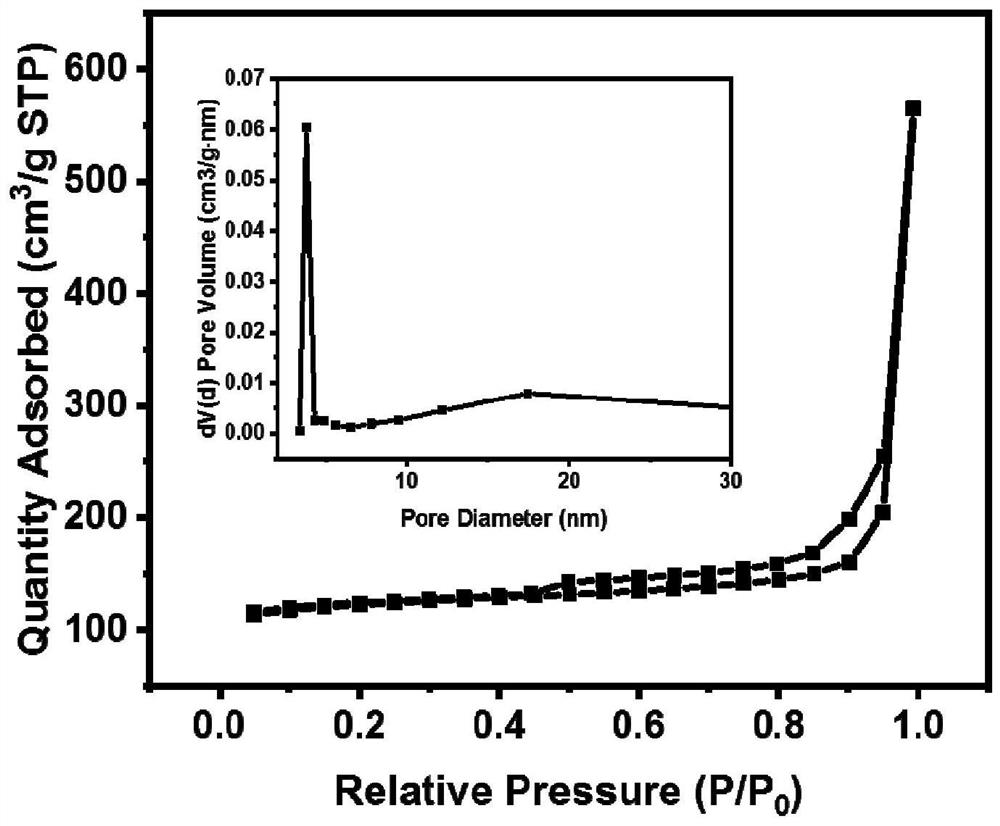
[0040] N of HP-Cu-BTC 2 Physical adsorption isotherm curves and pore size distribution diagrams, such as figure 2 As shown, its BET specific surface area is 380m 2 / g without CO 2 The BET specific surface area o...
Embodiment 3
[0046] 1. Preparation of HP-Cu-BTC
[0047] The preparation method of the hierarchically porous metal-organic framework material for drug-loaded sustained release of the present invention comprises the following steps:
[0048] (1) Take 1.2mL TX-100 and 2mL 1-butyl-3-methylimidazolium hexafluorophosphate successively into the reaction flask, mix and stir for 20min, then add 8mL ethylene glycol, and stir for 30min.
[0049] (2) 90mg of anhydrous copper acetate [Cu(OAC) 2 ], 52mg of trimesic acid were poured into the ionic liquid microemulsion in turn, stirred for 1-2min, transferred to a high-pressure reactor, and 5MPa CO 2 , Reaction 3h. After the reaction, it was washed with ethanol for three times, and dried in an oven at 60° C. to obtain HP-Cu-BTC after drying.
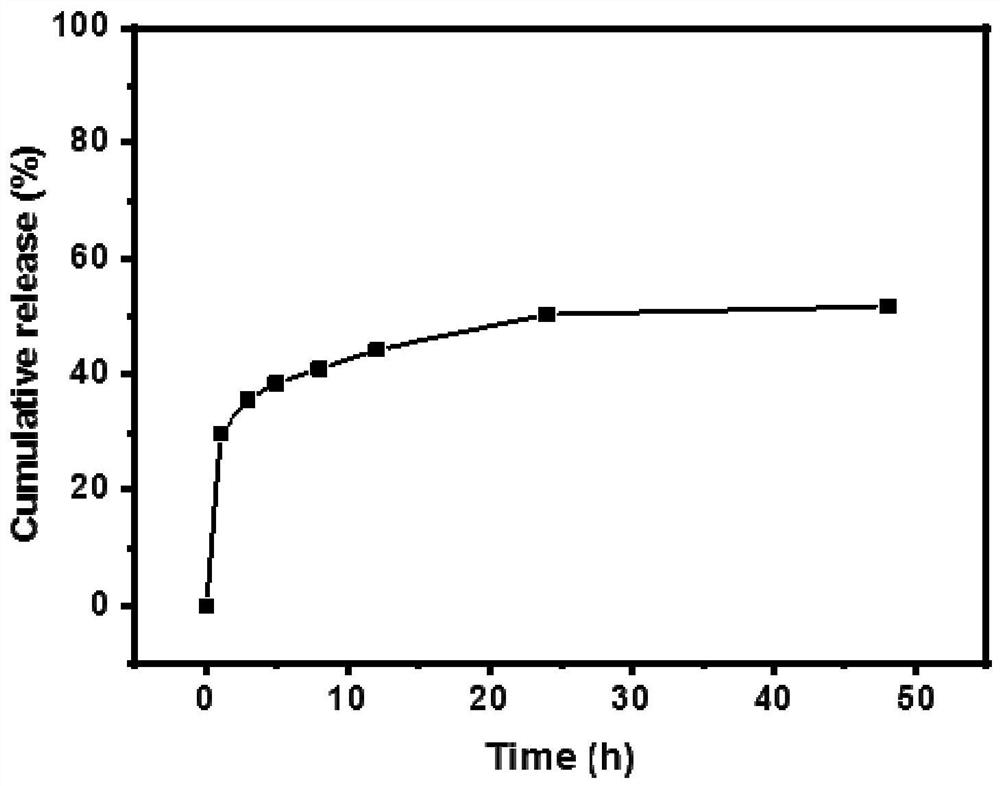
[0050] 2. Loading and sustained release test of HP-Cu-BTC
[0051] Further, 20 mg of HP-Cu-BTC was weighed and added to 2 mg / mL doxorubicin hydrochloride (DOX·HCl) methanol solution, stirred in the dark for 12 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com