GLP-2 analogs and peptibodies for administration before during, or after surgery
A GLP-2, post-operative technology, applied in the direction of peptides, hormone peptides, specific peptides, etc., can solve the problems that have not been studied and the potential of additional benefits of GLP-2 receptor agonists is not obvious
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
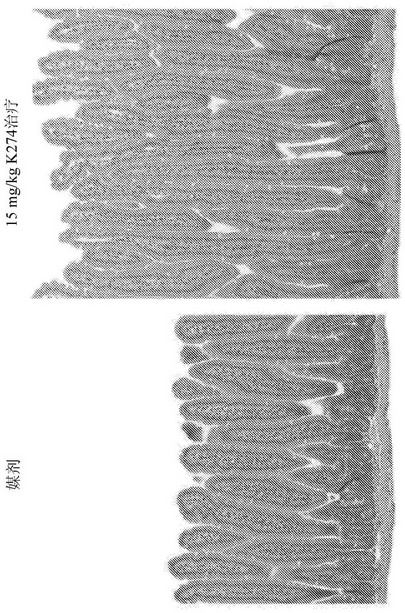
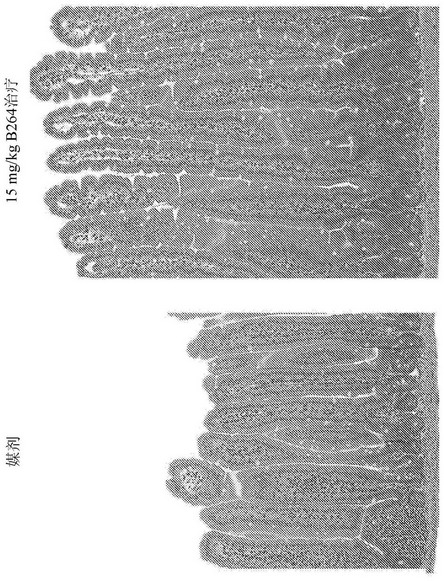
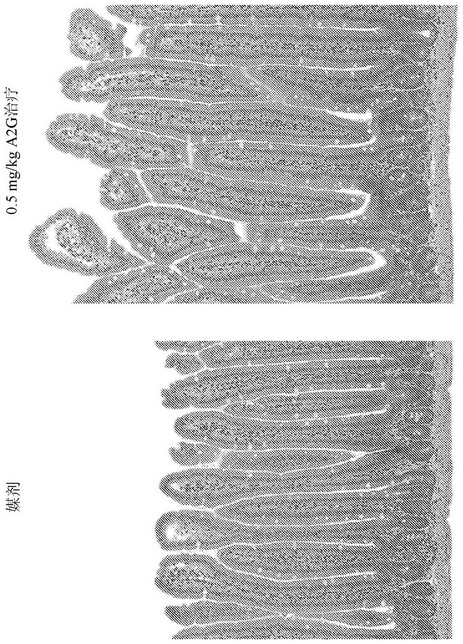
[0188] Example 1: Histological study of villus length and ductal depth in GLP-2 peptibody B264
[0189] Various doses of GLP-2 peptibody B264 were analyzed to assess the pharmacodynamic platform, primary endpoints were measurements of small intestine weight relative to total body weight and histological studies of villus length. Eleven groups of six female CD-1 mice each were formed. The groups are summarized in Table 1 below.
[0190] Table 1
[0191] Group
[0192] For histology, four micron paraffin sections were prepared for H&E and Ki67 IHC staining. After whole slide scanning, villi length and ductal depth were measured using a videograph, and Ki67 was analyzed. Antibodies against Ki67 are Rabbit antibody sold, catalog number ab 616667. Antibodies were used at a working concentration of 1:100 and Refine kit to detect. Ki67 staining results are shown in Figure 4 , 5A and 5B. The GLP-2 peptibody B264, GLP-2 peptibody K274, and GLP-2[A2G] peptides l...
example 2
[0193] Example 2: Administration of GLP-2 peptibodies to patients prior to surgery
[0194] Within a month, a patient with Crohn's disease was scheduled for small bowel resection surgery. It is expected that the patient will have a small bowel of 150 cm length after surgery. The small intestine is expected to remain continuous with the colon. The patient is expected to develop mild short bowel syndrome and will require parenteral nutritional support. At a dose of approximately 1.4 mg / kg, patients will be given weekly GLP-2 peptibodies were administered subcutaneously. Patients will be monitored for any side effects regarding digestion and intestinal absorption.
example 3
[0195] Example 3: Administration of teduglutide to a patient prior to surgery
[0196] Within a month, a patient with Crohn's disease was scheduled for small bowel resection surgery. It is expected that the patient will have a small bowel of 150 cm length after surgery. The small intestine is expected to remain continuous with the colon. The patient is expected to develop mild short bowel syndrome and will require parenteral nutritional support. Patients will be administered 0.05 mg / kgh(Gly2) intravenously daily as part of a treatment plan to reduce postoperative inflammation (a problem associated with Crohn's disease) and minimize the need for parenteral nutritional support GLP-2. Patients will be monitored for any side effects regarding digestion and intestinal absorption.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com