Pharmaceutical composition for treating and/or preventing ovarian cancer, and preparation method and application thereof
A composition and ovarian cancer technology, applied in the field of biomedicine, can solve the problems affecting the treatment compliance of ovarian cancer patients, the uncertainty of the effective rate of cisplatin treatment, and the high level of medical personnel's operation requirements, so as to make up for the digestive tract absorption barrier, Improve the effect of anticancer effect and improve the effect of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 The preparation of pharmaceutical composition of the present invention
[0034] According to the formulation in Table 1, mix cisplatin and alectinib in a container, and if necessary, stir at a high speed to obtain uniform and stable pharmaceutical compositions 1-4.
[0035] Table 1 pharmaceutical composition 1-4 formula
[0036] Numbering Cisplatin (mg) Alectinib (mg) Pharmaceutical Composition 1 2.8 0.1 Pharmaceutical composition 2 2.8 0.5 Pharmaceutical composition 3 2.8 1 Pharmaceutical composition 4 2.8 2
[0037] Note: Cisplatin was purchased from Shandong Qilu Pharmaceutical Factory; Alectinib was purchased from Roche Pharmaceuticals.
Embodiment 2
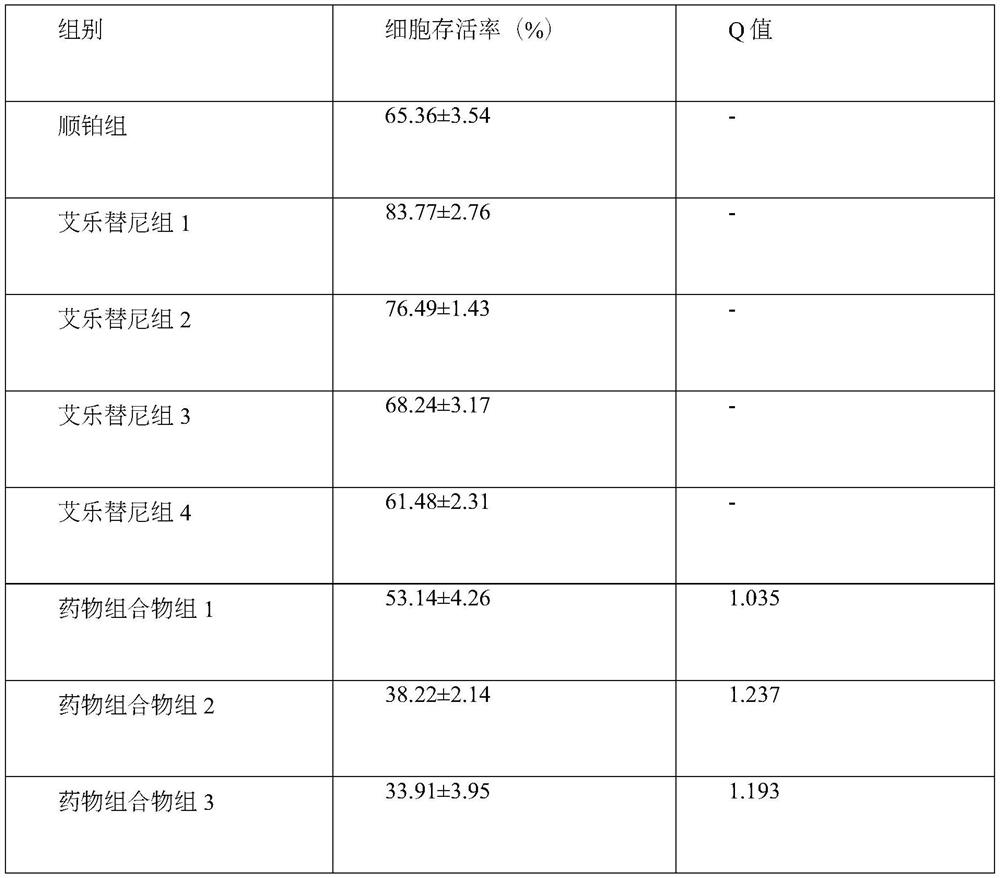
[0038] Example 2 Study on the Inhibitory Effect of A2780 Cell Proliferation
[0039] (1) Experimental materials:
[0040] A2780 (human ovarian cancer cell) cell line was purchased from Shanghai Zeye Biotechnology Co., Ltd.; Example 1 Pharmaceutical Composition 1-4.
[0041] (2) Experimental method:
[0042] Take the A2780 cells in the logarithmic growth phase, centrifuge to make a single cell suspension, and use 0.5×10 5 / mL inoculated in a 96-well plate with a volume of 200 μL per well. After inoculation, the culture plate was placed in a 37°C, 5% CO2 incubator and incubated for 24 hours until the cells were completely adhered to the wall.
[0043] An experimental group (cisplatin group, alectinib group, pharmaceutical composition group) and a control group (DMSO) were set. With DMSO as the dilution solvent, cisplatin group: cisplatin concentration 280mg / L; alectinib group 1-4: alectinib concentration 10mg / L, 50mg / L, 100mg / L, 200mg / L; pharmaceutical composition Groups...
Embodiment 3
[0052] Example 3 Effect of Ovarian Tumor Mass
[0053] 1. Experimental materials:
[0054] Healthy Kunming mice, male and female, 18-22g, were provided by the Experimental Animal Center of Nanjing University of Traditional Chinese Medicine.
[0055] A2780 (ovarian cancer cell) cell line was purchased from Shanghai Zeye Biotechnology Co., Ltd.; cisplatin was purchased from Shandong Qilu Pharmaceutical Factory; Alectinib was purchased from Roche Pharmaceuticals; Nigeria); icariin (purchased from Nantong Feiyu Biotechnology Co., Ltd.).
[0056] 2. Experimental method:
[0057] The mice were randomly divided into control group, model group, cisplatin group, alectinib group, icariin group, pharmaceutical composition 2 group, 90% pharmaceutical composition 2+10% icariin group according to body weight (weight ratio), 80% pharmaceutical composition 2+20% icariin group (weight ratio) and 60% pharmaceutical composition 2+40% icariin group, 10 in each group.
[0058] Culture human ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com